Population-based mechanistic prediction of oral drug absorption
- PMID: 19381840
- PMCID: PMC2691459
- DOI: 10.1208/s12248-009-9099-y
Population-based mechanistic prediction of oral drug absorption
Abstract
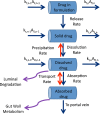
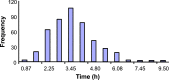
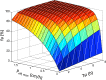
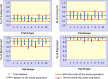
The bioavailability of drugs from oral formulations is influenced by many physiological factors including gastrointestinal fluid composition, pH and dynamics, transit and motility, and metabolism and transport, each of which may vary with age, gender, race, food, and disease. Therefore, oral bioavailability, particularly of poorly soluble and/or poorly permeable compounds and those that are extensively metabolized, often exhibits a high degree of inter- and intra-individual variability. While several models and algorithms have been developed to predict bioavailability in an average person, efforts to accommodate intrinsic variability in the component processes are less common. An approach that incorporates such variability for human populations within a mechanistic framework is described together with examples of its application to drug and formulation development.
Figures

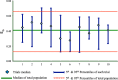
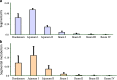
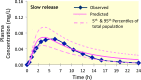
References
-
- Rostami-Hodjegan A, Tucker GT. Simulation and prediction of in vivo drug metabolism in human populations from in vitro data. Nat Rev Drug Discov. 2007;6(2):140–148. - PubMed
-
- Jamei M, Dickinson G, Rostami-Hodjegan A. A framework for assessing interindividual variability in pharmacokinetics using virtual human populations and integrating general knowledge of physical chemistry, biology, anatomy, physiology and genetics: a tale of ‘bottom-up’ vs ‘top-down’ recognition of covariates. Drug Metab Pharmacokinet. 2009;24(1):53–75. - PubMed
-
- Jamei M et al. The Simcyp® Population-Based ADME Simulator. Expert Opin Drug Metab Toxicol. 2009;5 2:211–23. - PubMed
-
- Yu LX, et al. Transport approaches to the biopharmaceutical design of oral drug delivery systems: prediction of intestinal absorption. Adv Drug Deliv Rev. 1996;19:359–376. - PubMed
-
- Yu LX, Crison JR, Amidon GL. Compartmental transit and dispersion model analysis of small intestinal transit flow in humans. Int J Pharm. 1996;140:111–118.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

