Insulin-like growth factor-I-stimulated Akt phosphorylation and oligodendrocyte progenitor cell survival require cholesterol-enriched membranes
- PMID: 19382214
- PMCID: PMC5513484
- DOI: 10.1002/jnr.22099
Insulin-like growth factor-I-stimulated Akt phosphorylation and oligodendrocyte progenitor cell survival require cholesterol-enriched membranes
Abstract
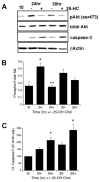
Previously we showed that insulin-like growth factor-I (IGF-I) promotes sustained phosphorylation of Akt in oligodendrocyte progenitor cells (OPCs) and that Akt phosphorylation is required for survival of these cells. The direct mechanisms, however, by which IGF-I promotes Akt phosphorylation are currently undefined. Recently, cholesterol-enriched membranes (CEMs) have been implicated in regulation of growth factor-mediated activation of the PI3K/Akt pathway and survival of mature oligodendrocytes; however, less is know about their role in OPC survival. In the present study, we investigate the role of CEMs in IGF-I-mediated Akt phosphorylation and OPC survival. We report that acute disruption of membrane cholesterol with methyl-beta-cyclodextrin results in altered OPC morphology and inhibition of IGF-I-mediated Akt phosphorylation. We also report that long-term inhibition of cholesterol biosynthesis with 25-hydroxycholesterol blocks IGF-I stimulated Akt phosphorylation and cell survival. Moreover, we show that the PI3K regulatory subunit, p85, Akt, and the IGF-IR are sequestered within cholesterol-enriched fractions in steady-state stimulation of the IGF-IR and that phosphorylated Akt and IGF-IR are present in cholesterol-enriched fractions with IGF-I stimulation. Together, the results of these studies support a role for CEMs or "lipid rafts" in IGF-I-mediated Akt phosphorylation and provide a better understanding of the mechanisms by which IGF-I promotes OPC survival.
Figures


Similar articles
-
Inhibition of Src-like kinases reveals Akt-dependent and -independent pathways in insulin-like growth factor I-mediated oligodendrocyte progenitor survival.J Biol Chem. 2005 Mar 11;280(10):8918-28. doi: 10.1074/jbc.M414267200. Epub 2005 Jan 4. J Biol Chem. 2005. PMID: 15632127
-
Insulin-like growth factor-1 provides protection against psychosine-induced apoptosis in cultured mouse oligodendrocyte progenitor cells using primarily the PI3K/Akt pathway.Mol Cell Neurosci. 2005 Nov;30(3):398-407. doi: 10.1016/j.mcn.2005.08.004. Epub 2005 Sep 19. Mol Cell Neurosci. 2005. PMID: 16169744
-
IGF-1 protects oligodendrocyte progenitors against TNFalpha-induced damage by activation of PI3K/Akt and interruption of the mitochondrial apoptotic pathway.Glia. 2007 Aug 15;55(11):1099-107. doi: 10.1002/glia.20530. Glia. 2007. PMID: 17577243
-
Membrane rafts segregate pro- from anti-apoptotic insulin-like growth factor-I receptor signaling in colon carcinoma cells stimulated by members of the tumor necrosis factor superfamily.Am J Pathol. 2005 Sep;167(3):761-73. doi: 10.1016/S0002-9440(10)62049-4. Am J Pathol. 2005. PMID: 16127155 Free PMC article.
-
Inhibition of the IGF-1-PI3K-Akt-mTORC2 pathway in lipid rafts increases neuronal vulnerability in a genetic lysosomal glycosphingolipidosis.Dis Model Mech. 2019 May 23;12(5):dmm036590. doi: 10.1242/dmm.036590. Dis Model Mech. 2019. PMID: 31036560 Free PMC article.
Cited by
-
Oligodendrocyte Response to Pathophysiological Conditions Triggered by Episode of Perinatal Hypoxia-Ischemia: Role of IGF-1 Secretion by Glial Cells.Mol Neurobiol. 2020 Oct;57(10):4250-4268. doi: 10.1007/s12035-020-02015-z. Epub 2020 Jul 21. Mol Neurobiol. 2020. PMID: 32691304 Free PMC article.
-
Sterol structure dependence of insulin receptor and insulin-like growth factor 1 receptor activation.Biochim Biophys Acta Biomembr. 2019 Apr 1;1861(4):819-826. doi: 10.1016/j.bbamem.2019.01.009. Epub 2019 Jan 22. Biochim Biophys Acta Biomembr. 2019. PMID: 30682326 Free PMC article.
-
R-Ras1 and R-Ras2 Are Essential for Oligodendrocyte Differentiation and Survival for Correct Myelination in the Central Nervous System.J Neurosci. 2018 May 30;38(22):5096-5110. doi: 10.1523/JNEUROSCI.3364-17.2018. Epub 2018 May 2. J Neurosci. 2018. PMID: 29720552 Free PMC article.
-
Neuron-targeted caveolin-1 improves neuromuscular function and extends survival in SOD1G93A mice.FASEB J. 2019 Jun;33(6):7545-7554. doi: 10.1096/fj.201802652RR. Epub 2019 Mar 20. FASEB J. 2019. PMID: 30894019 Free PMC article.
-
Integrin signaling in oligodendrocytes and its importance in CNS myelination.J Signal Transduct. 2011;2011:354091. doi: 10.1155/2011/354091. Epub 2010 Dec 20. J Signal Transduct. 2011. PMID: 21637375 Free PMC article.
References
-
- Baron W, Decker L, Colognato H, ffrench-Constant C. Regulation of integrin growth factor interactions in oligodendrocytes by lipid raft microdomains. Curr Biol. 2003;13:151–155. - PubMed
-
- Carson MJ, Behringer RR, Brinster RL, McMorris FA. Insulin-like growth factor I increases brain growth and central nervous system myelination in transgenic mice. Neuron. 1993;10:729–740. - PubMed
-
- Frederick TJ, Wood TL. IGF-I and FGF-2 coordinately enhance cyclin D1 and cyclin E-cdk2 association and activity to promote G1 progression in oligodendrocyte progenitor cells. Mol Cell Neurosci. 2004;25:480–492. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

