Applications of 1-alkenyl-1,1-heterobimetallics in the stereoselective synthesis of cyclopropylboronate esters, trisubstituted cyclopropanols and 2,3-disubstituted cyclobutanones
- PMID: 19382808
- PMCID: PMC2740478
- DOI: 10.1021/ja900147s
Applications of 1-alkenyl-1,1-heterobimetallics in the stereoselective synthesis of cyclopropylboronate esters, trisubstituted cyclopropanols and 2,3-disubstituted cyclobutanones
Abstract
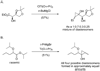
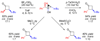
1-Alkenyl-1,1-heterobimetallics are potentially very useful in stereoselective organic synthesis but are relatively unexplored. Introduced herein is a practical application of 1-alkenyl-1,1-heterobimetallic intermediates in the synthesis of versatile cyclopropyl alcohol boronate esters, which are valuable building blocks. Thus, hydroboration of 1-alkynyl-1-boronate esters with dicyclohexylborane generates 1-alkenyl-1,1-diboro species. In situ transmetalation with dialkylzinc reagents furnishes 1-alkenyl-1,1-borozinc heterobimetallic intermediates. Addition of the more reactive ZnC bond to aldehydes generates the key B(pin) substituted allylic alkoxide intermediates. An in situ alkoxide directed cyclopropanation proceeds with the formation of two more CC bonds, affording cyclopropyl alcohol boronate esters with three new stereocenters in 58-89% isolated yields and excellent diastereoselectivities (>15:1 dr). Oxidation of the BC bond provides trisubstituted alpha-hydroxycyclopropyl carbinols as single diastereomers in good to excellent yields (75-93%). Facile pinacol-type rearrangement of the alpha-hydroxycyclopropyl carbinols provides access to both cis- and trans-2,3-disubstituted cyclobutanones with high stereoselectivity (>17:1 dr in most cases) from a common starting material. This methodology has been applied in the synthesis of quercus lactones A and B.
Figures


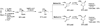



References
-
- Walsh PJ, Kozlwoski MC. Fundamentals of Asymmetric Catalysis. Sausalito, California: University Science Books; 2008. p. 688.
-
- Augustine RL, editor. Carbon-Carbon Bond Formation. Vol. 1. New York: Marcel Dekker, Inc; 1979. p. 461.
-
- Nógrádi M. Stereoselective Synthesis. New York: VCH Publishers, Inc; 1995. p. 370.
-
- Ho TL. Tandem Organic Reactions. New York: Wiley and Sons; 1992. p. 502.
-
- Wender PA, Miller BL. In: Organic Synthesis: Theory and Applications. Hudlicky T, editor. Vol. 2. Greenwich, CT: JAI Press; 1993. p. 27.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

