Multiple channel bridges for spinal cord injury: cellular characterization of host response
- PMID: 19382871
- PMCID: PMC2792065
- DOI: 10.1089/ten.TEA.2009.0081
Multiple channel bridges for spinal cord injury: cellular characterization of host response
Abstract
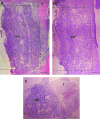
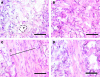
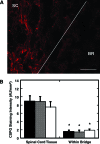
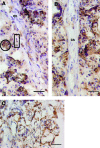
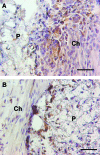
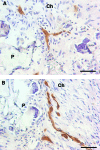
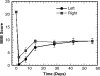
Bridges for treatment of the injured spinal cord must stabilize the injury site to prevent secondary damage and create a permissive environment that promotes regeneration. The host response to the bridge is central to creating a permissive environment, as the cell types that respond to the injury have the potential to secrete both stimulatory and inhibitory factors. We investigated multiple channel bridges for spinal cord regeneration and correlated the bridge structure to cell infiltration and axonal elongation. Poly(lactide-co-glycolide) bridges were fabricated by a gas foaming/particulate leaching process. Channels within the bridge had diameters of 150 or 250 microm, and the main body of the bridge was highly porous with a controllable pore size. Upon implantation in a rat spinal cord hemisection site, cells infiltrated into the bridge pores and channels, with the pore size influencing the rate of infiltration. The pores had significant cell infiltration, including fibroblasts, macrophages, S-100beta-positive cells, and endothelial cells. The channels of the bridge were completely infiltrated with cells, which had aligned axially, and consisted primarily of fibroblasts, S-100beta-positive cells, and endothelial cells. Reactive astrocytes were observed primarily outside of the bridge, and staining for chondroitin sulfate proteoglycans was decreased in the region surrounding the bridge relative to studies without bridges. Neurofilament staining revealed a preferential growth of the neural fibers within the bridge channels relative to the pores. Multiple channel bridges capable of supporting cellular infiltration, creating a permissive environment, and directing the growth of neural fibers have potential for promoting and directing spinal cord regeneration.
Figures




References
-
- Dusart I. Schwab M.E. Secondary cell death and the inflammatory reaction after dorsal hemisection of the rat spinal cord. Eur J Neurosci. 1994;6:712. - PubMed
-
- David S. Aguayo A.J. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science. 1981;214:931. - PubMed
-
- Stichel C.C. Muller H.W. Experimental strategies to promote axonal regeneration after traumatic central nervous system injury. Prog Neurobiol. 1998;56:119. - PubMed
-
- Schmidt C.E. Leach J.B. Neural tissue engineering: strategies for repair and regeneration. Annu Rev Biomed Eng. 2003;5:293. - PubMed
-
- Bunge M.B. Bridging areas of injury in the spinal cord. Neuroscientist. 2001;7:325. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

