Cardiac renewing: interstitial Cajal-like cells nurse cardiomyocyte progenitors in epicardial stem cell niches
- PMID: 19382895
- PMCID: PMC2737613
- DOI: 10.1111/j.1582-4934.2009.00758.x
Cardiac renewing: interstitial Cajal-like cells nurse cardiomyocyte progenitors in epicardial stem cell niches
Abstract
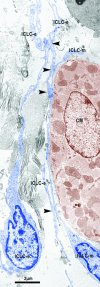
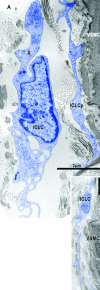
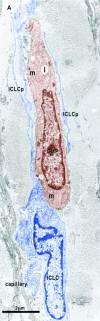
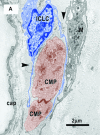
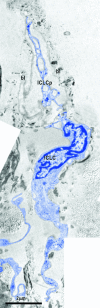
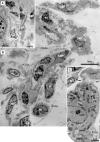
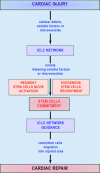
Recent studies suggested that various cell lineages exist within the subepicardium and we supposed that this area could host cardiac stem cell niches (CSCNs). Using transmission electron microscopy, we have found at least 10 types of cells coexisting in the subepicardium of normal adult mice: adipocytes, fibroblasts, Schwann cells and nerve fibres, isolated smooth muscle cells, mast cells, macrophages, lymphocytes, interstitial Cajal-like cells (ICLCs) and cardiomyocytes progenitors (CMPs). The latter cells, sited in the area of origin of coronary arteries and aorta, showed typical features of either very immature or developing cardiomyocytes. Some of these cells were connected to each other to form columns surrounded by a basal lamina and embedded in a cellular network made by ICLCs. Complex intercellular communication occurs between the ICLCs and CMPs through electron-dense nanostructures or through shed vesicles. We provide here for the first time the ultrastructural description of CSCN in the adult mice myocardium, mainly containing ICLCs and CMPs. The existence of resident CMPs in different developmental stages proves that cardiac renewing is a continuous process. We suggest that ICLCs might act as supporting nurse cells of the cardiac niches and may be responsible for activation, commitment and migration of the stem cells out of the niches. Briefly, not only resident cardiac stem cells but also ICLCs regulate myocyte turnover and contribute to both cardiac cellular homeostasis and endogenous repair/remodelling after injuries.
Figures














References
-
- Zhou B, Pu WT. More than a cover: epicardium as a novel source of cardiac progenitor cells. Regen Med. 2008;3:633–5. - PubMed
-
- Martin-Puig S, Wang Z, Chien KR. Lives of a heart cell: tracing the origins of cardiac progenitors. Cell Stem Cell. 2008;2:320–31. - PubMed
-
- Van Tuyn J, Atsma DE, Winter EM, Van Der Velde-van Dijke I, Pijnappels DA, Bax NA, Knaän-Shanzer S, Gittenberger-de Groot AC, Poelmann RE, Van Der Laarse A, Van Der Wall EE, Schalij MJ, De Vries AA. Epicardial cells of human adults can undergo an epithelial-to-mesenchymal transition and obtain characteristics of smooth muscle cells in vitro. Stem Cells. 2007;25:271–8. - PubMed
-
- Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, Qyang Y, Bu L, Sasaki M, Martin-Puig S, Sun Y, Evans SM, Laugwitz KL, Chien KR. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127:1151–65. - PubMed
-
- Laugwitz KL, Moretti A, Caron L, Nakano A, Chien KR. Islet1 cardiovascular progenitors: a single source for heart lineages. Development. 2008;135:193–205. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

