Phosphoprotein Keratin 23 accumulates in MSS but not MSI colon cancers in vivo and impacts viability and proliferation in vitro
- PMID: 19383294
- PMCID: PMC5543893
- DOI: 10.1016/j.molonc.2007.05.005
Phosphoprotein Keratin 23 accumulates in MSS but not MSI colon cancers in vivo and impacts viability and proliferation in vitro
Abstract
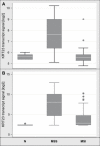
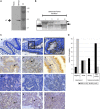
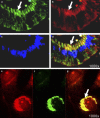
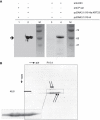
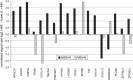
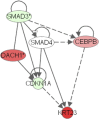
Transcript profiling of 27 normal colon mucosas and 258 adenocarcinomas showed Keratin23 to be increased in 78% microsatellite-stable tumors, while microsatellite-instable tumors showed low transcript levels, comparable to normal mucosas. Immunohistochemical analyses demonstrated that 88% of microsatellite-instable tumors were negative for Keratin23 protein, while 70% of MSS tumors and metastases derived from MSS-tumors showed high Keratin23 levels. Immunofluorescence analysis localized Keratin23 in the Golgi-apparatus. Golgi accumulation was unique for gastrointestinal adenocarcinomas. Immunoprecipitation and 2D-blot analysis revealed Keratin23 to be a 46.8 kDa phosphoprotein. Keratin23 impaired the proliferation of human colon cancer cells significantly, leading to cell death in microsatellite-instable but not microsatellite-stable cell lines, while COS7 cells experienced multiple nuclei and apoptosis. Keratin23 expression correlated significantly with transcription factor CEBPB. In conclusion, Keratin23 expression is a novel and important difference between microsatellite-stable and microsatellite-instable colon cancers.
Figures


References
-
- Benatti, P. , Gafa, R. , Barana, D. , Marino, M. , Scarselli, A. , Pedroni, M. , Maestri, I. , Guerzoni, L. , Roncucci, L. , Menigatti, M. , Roncari, B. , Maffei, S. , Rossi, G. , Ponti, G. , Santini, A. , Losi, L. , Di, G.C. , Oliani, C. , Ponz de, L.M. , Lanza, G. , 2005. Microsatellite instability and colorectal cancer prognosis. Clin. Cancer Res.. 11, 8332–8340. - PubMed
-
- Birkenkamp-Demtroder, K. , Christensen, L.L. , Olesen, S.H. , Frederiksen, C.M. , Laiho, P. , Aaltonen, L.A. , Laurberg, S. , Sorensen, F.B. , Hagemann, R. , Orntoft, T.F. , 2002. Gene expression in colorectal cancer. Cancer Res.. 62, 4352–4363. - PubMed
-
- Birkenkamp-Demtroder, K. , Wagner, L. , Sørensen, F.B. , Bording Astrup, L. , Gartner, W. , Scherubl, H. , Heine, B. , Christiansen, P. , Ørntoft, T.F. , 2005. Secretagogin is a novel marker for neuroendocrine differentiation. Neuroendocrinology. 82, 121–138. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

