Transitions between closed and open conformations of TolC: the effects of ions in simulations
- PMID: 19383457
- PMCID: PMC2718315
- DOI: 10.1016/j.bpj.2009.01.021
Transitions between closed and open conformations of TolC: the effects of ions in simulations
Abstract
Bacteria, such as Escherichia coli, use multidrug efflux pumps to export toxic substrates through their cell membranes. Upon formation of an efflux pump, the aperture of its outer membrane protein TolC opens and thereby enables the extrusion of substrate molecules. The specialty of TolC is its ability to dock to different transporters, making it a highly versatile export protein. Within this study, the transition between two conformations of TolC that are both available as crystal structures was investigated using all-atom molecular dynamics simulations. To create a partially open conformation from a closed one, the stability of the periplasmic aperture was weakened by a double point mutation at the constricting ring, which removes some salt bridges and hydrogen bonds. These mutants, which showed partial opening in previous experiments, did not spontaneously open during a 20-ns equilibration at physiological values of the KCl solution. Detailed analysis of the constricting ring revealed that the cations of the solvent were able to constitute ionic bonds in place of the removed salt bridges, which inhibited the opening of the aperture in simulations. To remove the ions from these binding positions within the available simulation time, an extra force was applied onto the ions. To keep the effect of this additional force rather flexible, it was applied in form of an artificial external electric field perpendicular to the membrane. Depending on the field direction and the ion concentration, these simulations led to a partial opening. In experiments, this energy barrier for the ions can be overcome by thermal fluctuations on a longer timescale.
Figures




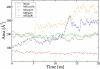
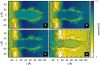



References
-
- Fernandes P. Antibacterial discovery and development—the failure of success? Nat. Biotechnol. 2006;24:1497–1503. - PubMed
-
- Lomovskaya O., Zgurskaya H.I., Totrov M., Watkins W.J. Waltzing transporters and “the dance macabre” between humans and bacteria. Nat. Rev. Drug Discov. 2007;6:56–65. - PubMed
-
- Alberts B. 2nd Ed. Garland Science; New York: 2004. Essential Cell Biology.
-
- Alberts B., Johnson A., Lewis J., Raff M., Roberts K. 4th Ed. Garland Science; New York: 2002. Molecular Biology of the Cell.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

