Lipid raft composition modulates sphingomyelinase activity and ceramide-induced membrane physical alterations
- PMID: 19383465
- PMCID: PMC2718262
- DOI: 10.1016/j.bpj.2008.12.3923
Lipid raft composition modulates sphingomyelinase activity and ceramide-induced membrane physical alterations
Abstract
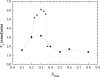
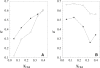
Lipid rafts and ceramide (Cer)-platforms are membrane domains that play an important role in several biological processes. Cer-platforms are commonly formed in the plasma membrane by the action of sphingomyelinase (SMase) upon hydrolysis of sphingomyelin (SM) within lipid rafts. The interplay among SMase activity, initial membrane properties (i.e., phase behavior and lipid lateral organization) and lipid composition, and the amount of product (Cer) generated, and how it modulates membrane properties were studied using fluorescence methodologies in model membranes. The activity of SMase was evaluated by following the hydrolysis of radioactive SM. It was observed that 1), the enzyme activity and extent of hydrolysis are strongly dependent on membrane physical properties but not on substrate content, and are higher in raft-like mixtures, i.e., mixtures with liquid-disordered/liquid-ordered phase separation; and 2), Cer-induced alterations are also dependent on membrane composition, specifically the cholesterol (Chol) content. In the lowest-Chol range, Cer segregates together with SM into small ( approximately 8.5 nm) Cer/SM-gel domains. With increasing Chol, the ability of Cer to recruit SM and form gel domains strongly decreases. In the high-Chol range, a Chol-enriched/SM-depleted liquid-ordered phase predominates. Together, these data suggest that in biological membranes, Chol in particular and raft domains in general play an important role in modulating SMase activity and regulating membrane physical properties by restraining Cer-induced alterations.
Figures
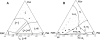
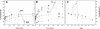





References
-
- Rajendran L., Simons K. Lipid rafts and membrane dynamics. J. Cell Sci. 2005;118:1099–1102. - PubMed
-
- Holthuis J.C.M., van Meer G., Huitema K. Lipid microdomains, lipid translocation and the organization of intracellular membrane transport. Mol. Membr. Biol. 2003;20:231–241. [Review] - PubMed
-
- Bollinger C.R., Teichgraber V., Gulbins E. Ceramide-enriched membrane domains. Biochim. Biophys. Acta. 2005;1746:284–294. - PubMed
-
- Gulbins E., Dreschers S., Wilker B., Grassme H. Ceramide, membrane rafts and infections. J. Mol. Med. 2004;82:357–363. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

