Obstacles on the microtubule reduce the processivity of Kinesin-1 in a minimal in vitro system and in cell extract
- PMID: 19383477
- PMCID: PMC2718299
- DOI: 10.1016/j.bpj.2009.01.015
Obstacles on the microtubule reduce the processivity of Kinesin-1 in a minimal in vitro system and in cell extract
Abstract
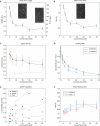
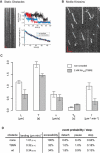
Inside cells, a multitude of molecular motors and other microtubule-associated proteins are expected to compete for binding to a limited number of binding sites available on microtubules. Little is known about how competition for binding sites affects the processivity of molecular motors and, therefore, cargo transport, organelle positioning, and microtubule organization, processes that all depend on the activity of more or less processive motors. Very few studies have been performed in the past to address this question directly. Most studies reported only minor effects of crowding on the velocity of motors. However, a controversy appears to exist regarding the effect of crowding on motor processivity. Here, we use single-molecule imaging of mGFP-labeled minimal dimeric kinesin-1 constructs in vitro to study the effects of competition on kinesin's processivity. For competitors, we use kinesin rigor mutants as static roadblocks, minimal wild-type kinesins as motile obstacles, and a cell extract as a complex mixture of microtubule-associated proteins. We find that mGFP-labeled kinesin-1 detaches prematurely from microtubules when it encounters obstacles, leading to a strong reduction of its processivity, a behavior that is largely independent of the type of obstacle used here. Kinesin has a low probability to wait briefly when encountering roadblocks. Our data suggest, furthermore, that kinesin can occasionally pass obstacles on the protofilament track.
Figures



Similar articles
-
Processive movement of single kinesins on crowded microtubules visualized using quantum dots.EMBO J. 2006 Jan 25;25(2):267-77. doi: 10.1038/sj.emboj.7600937. Epub 2006 Jan 12. EMBO J. 2006. PMID: 16407972 Free PMC article.
-
Kinesin's neck-linker determines its ability to navigate obstacles on the microtubule surface.Biophys J. 2014 Apr 15;106(8):1691-700. doi: 10.1016/j.bpj.2014.02.034. Biophys J. 2014. PMID: 24739168 Free PMC article.
-
Competition between kinesin-1 and myosin-V defines Drosophila posterior determination.Elife. 2020 Feb 14;9:e54216. doi: 10.7554/eLife.54216. Elife. 2020. PMID: 32057294 Free PMC article.
-
Move in for the kill: motile microtubule regulators.Trends Cell Biol. 2012 Nov;22(11):567-75. doi: 10.1016/j.tcb.2012.08.003. Epub 2012 Sep 6. Trends Cell Biol. 2012. PMID: 22959403 Free PMC article. Review.
-
Fluorescence microscopy applied to intracellular transport by microtubule motors.J Biosci. 2018 Jul;43(3):437-445. J Biosci. 2018. PMID: 30002263 Review.
Cited by
-
She1 affects dynein through direct interactions with the microtubule and the dynein microtubule-binding domain.Nat Commun. 2017 Dec 15;8(1):2151. doi: 10.1038/s41467-017-02004-2. Nat Commun. 2017. PMID: 29247176 Free PMC article.
-
Microtubule Defects Influence Kinesin-Based Transport In Vitro.Biophys J. 2016 May 24;110(10):2229-40. doi: 10.1016/j.bpj.2016.04.029. Biophys J. 2016. PMID: 27224488 Free PMC article.
-
Polycationic gold nanorods as multipurpose in vitro microtubule markers.Nanoscale Adv. 2020 Jul 13;2(9):4003-4010. doi: 10.1039/d0na00406e. eCollection 2020 Sep 16. Nanoscale Adv. 2020. PMID: 36132798 Free PMC article.
-
Modeling transport of extended interacting objects with drop-off phenomenon.PLoS One. 2022 May 2;17(5):e0267858. doi: 10.1371/journal.pone.0267858. eCollection 2022. PLoS One. 2022. PMID: 35499998 Free PMC article.
-
Head of myosin IX binds calmodulin and moves processively toward the plus-end of actin filaments.J Biol Chem. 2010 Aug 6;285(32):24933-42. doi: 10.1074/jbc.M110.101105. Epub 2010 Jun 10. J Biol Chem. 2010. PMID: 20538589 Free PMC article.
References
-
- Howard J. Sinauer Associates; Sunderland, MA: 2001. Mechanics of Motor Proteins and the Cytoskeleton.
-
- Nogales E. Structural insight into microtubule function. Annu. Rev. Biophys. Biomol. Struct. 2001;30:397–420. - PubMed
-
- Goldstein L.S., Philp A.V. The road less traveled: emerging principles of kinesin motor utilization. Annu. Rev. Cell Dev. Biol. 1999;15:141–183. - PubMed
-
- Gross S.P., Vershinin M., Shubeita G.T. Cargo transport: two motors are sometimes better than one. Curr. Biol. 2007;17:R478–R486. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

