Chaperones of F1-ATPase
- PMID: 19383603
- PMCID: PMC2719352
- DOI: 10.1074/jbc.M109.002568
Chaperones of F1-ATPase
Abstract
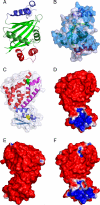
Mitochondrial F(1)-ATPase contains a hexamer of alternating alpha and beta subunits. The assembly of this structure requires two specialized chaperones, Atp11p and Atp12p, that bind transiently to beta and alpha. In the absence of Atp11p and Atp12p, the hexamer is not formed, and alpha and beta precipitate as large insoluble aggregates. An early model for the mechanism of chaperone-mediated F(1) assembly (Wang, Z. G., Sheluho, D., Gatti, D. L., and Ackerman, S. H. (2000) EMBO J. 19, 1486-1493) hypothesized that the chaperones themselves look very much like the alpha and beta subunits, and proposed an exchange of Atp11p for alpha and of Atp12p for beta; the driving force for the exchange was expected to be a higher affinity of alpha and beta for each other than for the respective chaperone partners. One important feature of this model was the prediction that as long as Atp11p is bound to beta and Atp12p is bound to alpha, the two F(1) subunits cannot interact at either the catalytic site or the noncatalytic site interface. Here we present the structures of Atp11p from Candida glabrata and Atp12p from Paracoccus denitrificans, and we show that some features of the Wang model are correct, namely that binding of the chaperones to alpha and beta prevents further interactions between these F(1) subunits. However, Atp11p and Atp12p do not resemble alpha or beta, and it is instead the F(1) gamma subunit that initiates the release of the chaperones from alpha and beta and their further assembly into the mature complex.
Figures






References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

