State-stabilizing Interactions in Bacterial Mechanosensitive Channel Gating and Adaptation
- PMID: 19383606
- PMCID: PMC2740535
- DOI: 10.1074/jbc.R109.009357
State-stabilizing Interactions in Bacterial Mechanosensitive Channel Gating and Adaptation
Abstract
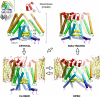
We outline several principles that we believe define the gating of two bacterial mechanosensitive channels, MscL and MscS. Serving as turgor regulators in bacteria and other walled cells, these molecules are tangible models for studying conformational transitions in membrane proteins driven directly by membrane tension. MscL, a compact pentamer, reversibly opens a gigantic 30-A pore at near-lytic tensions. MscS, a heptameric complex, exhibits transient activation of a smaller pore at moderate tensions, thereby entering a tension-insensitive inactivated state. By comparing the structures and predicted transitions in these channels, we concluded that opening is commonly achieved through tilting and outward motion of the pore-lining helices, which is kinetically limited by hydration of the pore. The intricate adaptive behavior in MscS appears to depend on specific interhelical associations and the flexibility of the pore-lining helices. We discuss physical factors that may direct the transitions and stabilize main functional states in these channels.
Figures
Similar articles
-
Gating of MscL studied by steered molecular dynamics.Biophys J. 2003 Oct;85(4):2087-99. doi: 10.1016/S0006-3495(03)74637-2. Biophys J. 2003. PMID: 14507677 Free PMC article.
-
An in vivo assay identifies changes in residue accessibility on mechanosensitive channel gating.Proc Natl Acad Sci U S A. 2004 Jul 6;101(27):10161-5. doi: 10.1073/pnas.0402040101. Epub 2004 Jun 28. Proc Natl Acad Sci U S A. 2004. PMID: 15226501 Free PMC article.
-
Hydration properties of mechanosensitive channel pores define the energetics of gating.J Phys Condens Matter. 2010 Nov 17;22(45):454120. doi: 10.1088/0953-8984/22/45/454120. Epub 2010 Oct 29. J Phys Condens Matter. 2010. PMID: 21339607
-
MscS, the bacterial mechanosensitive channel of small conductance.Int J Biochem Cell Biol. 2008;40(4):581-5. doi: 10.1016/j.biocel.2007.03.013. Epub 2007 Mar 24. Int J Biochem Cell Biol. 2008. PMID: 17466568 Review.
-
Gating the bacterial mechanosensitive channels: MscS a new paradigm?Curr Opin Microbiol. 2004 Apr;7(2):163-7. doi: 10.1016/j.mib.2004.02.006. Curr Opin Microbiol. 2004. PMID: 15063854 Review.
Cited by
-
Luminescence resonance energy transfer in the cytoplasm of live Escherichia coli cells.Biochemistry. 2011 Aug 16;50(32):6789-96. doi: 10.1021/bi200779u. Epub 2011 Jul 21. Biochemistry. 2011. PMID: 21739954 Free PMC article.
-
Structures of membrane proteins.Q Rev Biophys. 2010 Feb;43(1):65-158. doi: 10.1017/S0033583510000041. Q Rev Biophys. 2010. PMID: 20667175 Free PMC article. Review.
-
Unconventional mechanics of lipid membranes: a potential role for mechanotransduction of hair cell stereocilia.Biophys J. 2015 Feb 3;108(3):610-21. doi: 10.1016/j.bpj.2014.12.029. Biophys J. 2015. PMID: 25650928 Free PMC article.
-
Bacterial mechanosensitive channels--MscS: evolution's solution to creating sensitivity in function.Annu Rev Biophys. 2012;41:157-77. doi: 10.1146/annurev-biophys-101211-113227. Epub 2012 Feb 23. Annu Rev Biophys. 2012. PMID: 22404681 Free PMC article. Review.
-
Curvature generation and pressure profile modulation in membrane by lysolipids: insights from coarse-grained simulations.Biophys J. 2009 Oct 21;97(8):2267-76. doi: 10.1016/j.bpj.2009.07.051. Biophys J. 2009. PMID: 19843459 Free PMC article.
References
-
- Wood J. M., Bremer E., Csonka L. N., Kraemer R., Poolman B., van der Heide T., Smith L. T. (2001) Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 130, 437–460 - PubMed
-
- Perozo E., Rees D. C. (2003) Curr. Opin. Struct. Biol. 13, 432–442 - PubMed
-
- Anishkin A., Kung C. (2005) Curr. Opin. Neurobiol. 15, 397–405 - PubMed
-
- Sukharev S. I., Blount P., Martinac B., Blattner F. R., Kung C. (1994) Nature 368, 265–268 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

