Multicopy suppressor analysis of thermosensitive YIP1 alleles implicates GOT1 in transport from the ER
- PMID: 19383723
- PMCID: PMC2680100
- DOI: 10.1242/jcs.042457
Multicopy suppressor analysis of thermosensitive YIP1 alleles implicates GOT1 in transport from the ER
Abstract
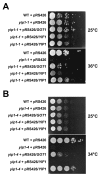
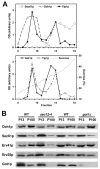
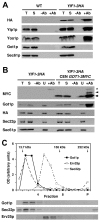
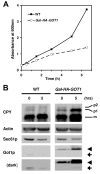
Yip1p belongs to a conserved family of membrane-spanning proteins that are involved in intracellular trafficking. Studies have shown that Yip1p forms a heteromeric integral membrane complex, is required for biogenesis of ER-derived COPII vesicles, and can interact with Rab GTPases. However, the role of the Yip1 complex in vesicle budding is not well understood. To gain further insight, we isolated multicopy suppressors of the thermosensitive yip1-2 allele. This screen identified GOT1, FYV8 and TSC3 as novel high-copy suppressors. The strongest suppressor, GOT1, also displayed moderate suppressor activity toward temperature-sensitive mutations in the SEC23 and SEC31 genes, which encode subunits of the COPII coat. Further characterization of Got1p revealed that this protein was efficiently packaged into COPII vesicles and cycled rapidly between the ER and Golgi compartments. Based on the findings we propose that Got1p has an unexpected role in vesicle formation from the ER by influencing membrane properties.
Figures




Similar articles
-
Yos1p is a novel subunit of the Yip1p-Yif1p complex and is required for transport between the endoplasmic reticulum and the Golgi complex.Mol Biol Cell. 2005 Apr;16(4):1673-83. doi: 10.1091/mbc.e04-10-0873. Epub 2005 Jan 19. Mol Biol Cell. 2005. PMID: 15659647 Free PMC article.
-
A role for Yip1p in COPII vesicle biogenesis.J Cell Biol. 2003 Oct 13;163(1):57-69. doi: 10.1083/jcb.200306118. J Cell Biol. 2003. PMID: 14557247 Free PMC article.
-
TRAPPI tethers COPII vesicles by binding the coat subunit Sec23.Nature. 2007 Feb 22;445(7130):941-4. doi: 10.1038/nature05527. Epub 2007 Feb 7. Nature. 2007. PMID: 17287728
-
The highly conserved COPII coat complex sorts cargo from the endoplasmic reticulum and targets it to the golgi.Cold Spring Harb Perspect Biol. 2013 Feb 1;5(2):a013367. doi: 10.1101/cshperspect.a013367. Cold Spring Harb Perspect Biol. 2013. PMID: 23378591 Free PMC article. Review.
-
New insights into the structural mechanisms of the COPII coat.Traffic. 2010 Mar;11(3):303-10. doi: 10.1111/j.1600-0854.2009.01026.x. Epub 2009 Dec 7. Traffic. 2010. PMID: 20070605 Review.
Cited by
-
Genecentric: a package to uncover graph-theoretic structure in high-throughput epistasis data.BMC Bioinformatics. 2013 Jan 18;14:23. doi: 10.1186/1471-2105-14-23. BMC Bioinformatics. 2013. PMID: 23331614 Free PMC article.
-
GOLGI TRANSPORT 1B Regulates Protein Export from the Endoplasmic Reticulum in Rice Endosperm Cells.Plant Cell. 2016 Nov;28(11):2850-2865. doi: 10.1105/tpc.16.00717. Epub 2016 Nov 1. Plant Cell. 2016. PMID: 27803308 Free PMC article.
-
Characteristics and Functions of the Yip1 Domain Family (YIPF), Multi-Span Transmembrane Proteins Mainly Localized to the Golgi Apparatus.Front Cell Dev Biol. 2019 Jul 30;7:130. doi: 10.3389/fcell.2019.00130. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31417902 Free PMC article. Review.
-
Mining Data From Plasma Cell Differentiation Identified Novel Genes for Engineering of a Yeast Antibody Factory.Front Bioeng Biotechnol. 2020 Mar 31;8:255. doi: 10.3389/fbioe.2020.00255. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32296695 Free PMC article.
-
A novel automated image analysis pipeline for quantifying morphological changes to the endoplasmic reticulum in cultured human cells.BMC Bioinformatics. 2021 Sep 8;22(1):427. doi: 10.1186/s12859-021-04334-x. BMC Bioinformatics. 2021. PMID: 34496765 Free PMC article.
References
-
- Ausubel, R. M., Brent, R., Kingston, R. E., Moore, D. D., Seidman, J. G., Smith, J. A. and Struhl, K. (1987). Current Protocols in Molecular Biology. New York: Greene Publishing and Wiley-Interscience.
-
- Baetz, K. K., Krogan, N. J., Emili, A., Greenblatt, J. and Hieter, P. (2004). The ctf13-30/CTF13 genomic haploinsufficiency modifier screen identifies the yeast chromatin remodeling complex RSC, which is required for the establishment of sister chromatid cohesion. Mol. Cell. Biol. 24, 1232-1244. - PMC - PubMed
-
- Baker, D., Hicke, L., Rexach, M., Schleyer, M. and Schekman, R. (1988). Reconstitution of SEC gene product-dependent intercompartmental protein transport. Cell 54, 335-344. - PubMed
-
- Banfield, D. K., Lewis, M. J. and Pelham, H. R. (1995). A SNARE-like protein required for traffic through the Golgi complex. Nature 375, 806-809. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

