The steroid receptor coactivator-1 regulates twist expression and promotes breast cancer metastasis
- PMID: 19383905
- PMCID: PMC2911143
- DOI: 10.1158/0008-5472.CAN-08-4389
The steroid receptor coactivator-1 regulates twist expression and promotes breast cancer metastasis
Abstract
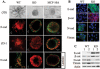
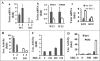
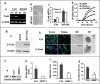
In breast cancer, steroid receptor coactivator-1 (SRC-1) expression positively correlates with HER2 expression and poor prognosis. In mouse mammary tumor virus-polyoma middle T (PyMT) breast cancer mouse model, SRC-1 strongly promotes mammary tumor metastasis. However, the molecular targets and mechanisms that mediate the role of SRC-1 in metastasis are unknown. In this study, SRC-1 wild-type (WT) and knockout (KO) cell lines were developed from the mammary tumors of WT/PyMT and KO/PyMT mice. WT cells exhibited strong migration and invasion capabilities, reduced E-cadherin and beta-catenin epithelial markers, gained N-cadherin and vimentin mesenchymal markers, and formed undifferentiated invasive structures in three-dimensional culture. In contrast, KO cells showed slow migration and invasion, retained E-cadherin, had less N-cadherin and vimentin, and developed partially differentiated three-dimensional structures. Importantly, WT cells expressed Twist, a master regulator of metastasis, at significantly higher levels versus KO cells. SRC-1 knockdown in WT cells reduced Twist expression, whereas SRC-1 restoration in KO cells also rescued Twist expression. Furthermore, SRC-1 was found to coactivate Twist transcription through physical interaction with the transcription factor PEA3 at the proximal Twist promoter. Accordingly, Twist knockdown in WT cells increased E-cadherin and reduced cell invasion and metastasis, and Twist expression in KO cells decreased E-cadherin and increased cell invasion. SRC-1 knockdown in human breast cancer cells also decreased Twist, cell migration, and invasion. Therefore, SRC-1 promotes breast cancer invasiveness and metastasis by coactivating PEA3-mediated Twist expression. Intervention of SRC-1 function may provide new strategies to inhibit breast cancer metastasis.
Figures



References
-
- Kauffman EC, Robinson VL, Stadler WM, Sokoloff MH, Rinker-Schaeffer CW. Metastasis suppression: the evolving role of metastasis suppressor genes for regulating cancer cell growth at the secondary site. J Urol. 2003;169:1122–1133. - PubMed
-
- Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. - PubMed
-
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. - PubMed
-
- Moustafa AS, Nicolson GL. Breast cancer metastasis-associated genes: prognostic significance and therapeutic implications. Oncol Res. 1997;9:505–525. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

