Polyphenolic profile and bioactivity study of Oenothera speciosa Nutt. aerial parts
- PMID: 19384277
- PMCID: PMC6254166
- DOI: 10.3390/molecules14041456
Polyphenolic profile and bioactivity study of Oenothera speciosa Nutt. aerial parts
Abstract
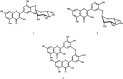
Two new flavonol glycosides, myricetin 4'-O-alpha-L-rhamnopyranoside (1) and quercetin 3'-O-alpha-L-rhamnopyranoside (2), together with a novel biflavonol compound, speciin (3), as well as eleven phenolic metabolites, namely myricitrin (4), europetin 3-O-alpha-L-(1)C(4)-rhamnopyranoside (5), quercitrin (6), hyperin (7), rhamnetin 3-O-beta-galacto-pyranoside (8), caffeic acid (9), caffeic acid methyl ester (10), chlorogenic acid (11), chlorogenic acid methyl ester (12), gallic acid (13) and gallic acid methyl ester (14), were identified from the 80 % methanol extract of the aerial parts (leaves and stems) of Oenothera speciosa Nutt. (Onagraceae). In addition myricetin (15), quercetin (16) and ellagic acid (17) were identified from the chloroform extract. The structures were established depending on their chemical and physical analyses (UV, HR-ESIMS, 1D and 2D NMR). It was found that 80 % aqueous methanol extract of O. speciosa is non-toxic to mice up to 5 g kg(-1)b wt. The investigated extract exhibited significant antihyperglycaemic and anti-inflammatory activities in a dose dependant manner. Also, the 80 % methanol extract, myricitrin(4) and hyperin(7) showed potent antioxidant activity in vitro using 1,1-diphenyl 2-picryl hydrazyl (DPPH) radical assay.
Figures

References
-
- Bailey L.H. Manual of cultivated plants. Revised Edition. Revised Edition. The Macmillan Company; New York, USA: 1958. pp. 733–739.
-
- Lawrence G.H.M. Taxonomy of vascular plants. The Macmillan Company; New York, USA: 1958. pp. 637–639.
-
- Hudson B.J.F. Evening primrose (Oenothera spp.) oil and seed. J. Am. Oil Chem. Soc. 1984;61:540–543. doi: 10.1007/BF02677026. - DOI
-
- Michimasa H. Antioxidant and antiaging effect of Evening Primrose seed polyphenols. Fragr. J. 2004;32:82–87.
-
- Yoshida T., Hatano T., Chou T., Yasuhara T., Matsuda M., Yazaki K., Okuda T., Miyamoto K., Koshiura R., Nitta A. Antitumor hydrolysable tannin oligomers with macrocyclic structures. Tennen Yuki Kagobutsu Toronkai Koen Yoshishu. 1990;32:221–228.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

