Temozolomide with radiation therapy in high grade brain gliomas: pharmaceuticals considerations and efficacy; a review article
- PMID: 19384285
- PMCID: PMC6254280
- DOI: 10.3390/molecules14041561
Temozolomide with radiation therapy in high grade brain gliomas: pharmaceuticals considerations and efficacy; a review article
Abstract
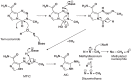
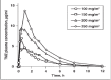
Malignant gliomas (glioblastoma multiforme and anaplastic astrocytoma) which have a combined incidence of 5-8/100,000 population, represent the most common primary central nervous system tumors. The treatment outcomes even with aggressive approach including surgery, radiation therapy and chemotherapy are dismal with median reported survival is less than 1 year. Temozolomide is a new drug which has shown promise in treating malignant gliomas and other difficult-to-treat tumors. This drug is a per os (p.o) imidazotetrazine second-generation alkylating agent which represents the leading compound in a new class of chemotherapeutic agents that enter the cerebrospinal fluid and do not require hepatic metabolism for activation. The efficacy of temozolomide was tested in vitro studies and has demonstrated schedule-dependent antitumor activity against highly resistant malignancies, including high-grade glioma (HGG). In addition, in clinical studies, temozolomide consistently demonstrates reproducible linear pharmacokinetics with approximately 100% p.o. bioavailability, noncumulative minimal myelosuppression that is rapidly reversible, and activity against a variety of solid tumors in both children and adults. Moreover, preclinical studies have evaluated the combination of temozolomide with other alkylating agents and inhibitors of the DNA repair protein O(6)-alkylguanine alkyltransferase to overcome resistance to chemotherapy in malignant glioma and malignant metastatic melanoma. At the present time temozolomide is approved in the United States for the treatment of adult patients with refractory anaplastic astrocytoma and, in the European Union, for treatment of glioblastoma multiforme showing progression or recurrence after standard therapy. Temozolomide's characteristics which make it a candidate for a wide range of clinical testing to evaluate the potential of combination treatments in different tumor types are its predictable bioavailability and minimal toxicity. An overview of the mechanism of action of temozolomide and a summary of results from more important randomized controlled clinical trials in high grade gliomas are presented here.
Figures
References
-
- O’Reilly S.M., Newlands E.S., Glaser M.G., Brampton M., Rice-Edwards J.M., Illingworth R.D., Richards P.G., Kennard C., Colquhoun I.R., Lewis P. Temozolomide: A new cytotoxic agent with promising activity against primary brain tumors. Eur. J. Cancer. 1993;29:940–942. doi: 10.1016/S0959-8049(05)80198-4. - DOI - PubMed
-
- Newlands E.S., O’Reilly S.M., Glaser M.G., Bower M., Evans H., Brock C., Brampton M.H., Colquhoun I., Lewis P., Rice-Edwards J.M., Illingworth R., Richards P.G. The Charing Cross Hospital experience with temozolomide in patients with gliomas. Eur. J. Cancer. 1996;32A:2236–2241. - PubMed
-
- Yung W.K., Prados M.D., Yaya-Tur R., Rosenfeld S.S., Brada M., Friedman H.S., AlbrightR. , Olson J., Chang S.M., O’Neill A.M., Friedman A.H., Bruner J., Yue N., Dugan M., Zaknoen S., Levin V.A. Multicenter phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. J. Clin. Oncol. 1999;17:2762–2771. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical