Selecting promising treatments in randomized Phase II cancer trials with an active control
- PMID: 19384691
- PMCID: PMC2896482
- DOI: 10.1080/10543400902802425
Selecting promising treatments in randomized Phase II cancer trials with an active control
Abstract
The primary objective of Phase II cancer trials is to evaluate the potential efficacy of a new regimen in terms of its antitumor activity in a given type of cancer. Due to advances in oncology therapeutics and heterogeneity in the patient population, such evaluation can be interpreted objectively only in the presence of a prospective control group of an active standard treatment. This paper deals with the design problem of Phase II selection trials in which several experimental regimens are compared to an active control, with an objective to identify an experimental arm that is more effective than the control or to declare futility if no such treatment exists. Conducting a multi-arm randomized selection trial is a useful strategy to prioritize experimental treatments for further testing when many candidates are available, but the sample size required in such a trial with an active control could raise feasibility concerns. In this study, we extend the sequential probability ratio test for normal observations to the multi-arm selection setting. The proposed methods, allowing frequent interim monitoring, offer high likelihood of early trial termination, and as such enhance enrollment feasibility. The termination and selection criteria have closed form solutions and are easy to compute with respect to any given set of error constraints. The proposed methods are applied to design a selection trial in which combinations of sorafenib and erlotinib are compared to a control group in patients with non-small-cell lung cancer using a continuous endpoint of change in tumor size. The operating characteristics of the proposed methods are compared to that of a single-stage design via simulations: The sample size requirement is reduced substantially and is feasible at an early stage of drug development.
Figures
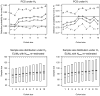
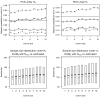
References
-
- Bischoff W, Miller F. Adaptive two-stage test procedures to find the best treatment in clinical trials. Biometrika. 2005;92:197–212.
-
- Cheung YK. Simple sequential boundaries for treatment selection in multi-armed randomized clinical trials with a control. Biometrics. 2008 in press. - PubMed
-
- Cheung YK, Gordon PH, Levin B. Selecting promising ALS therapies in clinical trials. Neurology. 2006;67:1748–1751. - PubMed
-
- Hochberg Y, Tamhane AC. Multiple Comparison Procedures. Wiley; 1987.
-
- Jung SH. Randomized phase II trials with a prospective control. Statistics in Medicine. 2007 in press. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
