Increased Hox activity mimics the teratogenic effects of excess retinoic acid signaling
- PMID: 19384962
- PMCID: PMC2739864
- DOI: 10.1002/dvdy.21951
Increased Hox activity mimics the teratogenic effects of excess retinoic acid signaling
Abstract
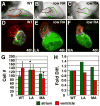
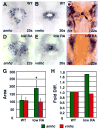
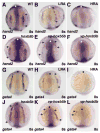
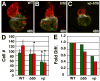
Excess retinoic acid (RA) signaling can be teratogenic and result in cardiac birth defects, but the cellular and molecular origins of these defects are not well understood. Excessive RA signaling can completely eliminate heart formation in the zebrafish embryo. However, atrial and ventricular cells are differentially sensitive to more modest increases in RA signaling. Increased Hox activity, downstream of RA signaling, causes phenotypes similar to those resulting from excess RA. These results suggest that Hox activity mediates the differential effects of ectopic RA on atrial and ventricular cardiomyocytes and may underlie the teratogenic effects of RA on the heart.
Figures


References
-
- Berdougo E, Coleman H, Lee DH, Stainier DY, Yelon D. Mutation of weak atrium/atrial myosin heavy chain disrupts atrial function and influences ventricular morphogenesis in zebrafish. Development. 2003;130:6121–6129. - PubMed
-
- Bruce AE, Oates AC, Prince VE, Ho RK. Additional hox clusters in the zebrafish: divergent expression patterns belie equivalent activities of duplicate hoxB5 genes. Evol Dev. 2001;3:127–144. - PubMed
-
- Hochgreb T, Linhares VL, Menezes DC, Sampaio AC, Yan CY, Cardoso WV, Rosenthal N, Xavier-Neto J. A caudorostral wave of RALDH2 conveys anteroposterior information to the cardiac field. Development. 2003;130:5363–5374. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

