Target-specific contrast agents for magnetic resonance microscopy
- PMID: 19385012
- PMCID: PMC2762731
- DOI: 10.1016/j.neuroimage.2009.01.030
Target-specific contrast agents for magnetic resonance microscopy
Abstract
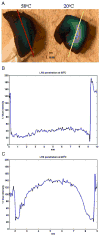
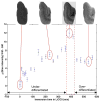
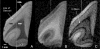
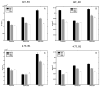
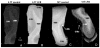
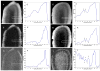
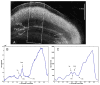
High-resolution ex vivo magnetic resonance (MR) imaging can be used to delineate prominent architectonic features in the human brain, but increased contrast is required to visualize more subtle distinctions. To aid MR sensitivity to cell density and myelination, we have begun the development of target-specific paramagnetic contrast agents. This work details the first application of luxol fast blue (LFB), an optical stain for myelin, as a white matter-selective MR contrast agent for human ex vivo brain tissue. Formalin-fixed human visual cortex was imaged with an isotropic resolution between 80 and 150 microm at 4.7 and 14 T before and after en bloc staining with LFB. Longitudinal (R1) and transverse (R2) relaxation rates in LFB-stained tissue increased proportionally with myelination at both field strengths. Changes in R1 resulted in larger contrast-to-noise ratios (CNR), per unit time, on T1-weighted images between more myelinated cortical layers (IV-VI) and adjacent, superficial layers (I-III) at both field strengths. Specifically, CNR for LFB-treated samples increased by 229 +/- 13% at 4.7 T and 269 +/- 25% at 14 T when compared to controls. Also, additional cortical layers (IVca, IVd, and Va) were resolvable in 14 T-MR images of LFB-treated samples but not in control samples. After imaging, samples were sliced in 40-micron sections, mounted, and photographed. Both the macroscopic and microscopic distributions of LFB were found to mimic those of traditional histological preparations. Our results suggest target-specific contrast agents will enable more detailed MR images with applications in imaging pathological ex vivo samples and constructing better MR atlases from ex vivo brains.
Figures


References
-
- Annese J, Pitiot A, Dinov ID, Toga AW. A myelo-architectonic method for the structural classification of cortical areas. Neuroimage. 2004;21 (1):15–26. - PubMed
-
- Barbier EL, Marrett S, Danek A, Vortmeyer A, van Gelderen P, Duyn J, Bandettini P, Grafman J, Koretsky AP. Imaging cortical anatomy by high-resolution MR at 3.0 T: detection of the stripe of Gennari in visual area 17. Magn Reson Med. 2002;48:735–738. - PubMed
-
- Blamire AM, Rowe JG, Styles P, McDonald B. Optimising imaging parameters for post mortem MR imaging of the human brain. Acta Radiol. 1999;40:593–597. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U24 RR021382/RR/NCRR NIH HHS/United States
- P41-RR14075/RR/NCRR NIH HHS/United States
- R01EB006758/EB/NIBIB NIH HHS/United States
- R01 NS052585-01/NS/NINDS NIH HHS/United States
- R01 EB001550/EB/NIBIB NIH HHS/United States
- U54 EB005149/EB/NIBIB NIH HHS/United States
- P41 RR014075/RR/NCRR NIH HHS/United States
- K25 AG029415/AG/NIA NIH HHS/United States
- R01 EB006758/EB/NIBIB NIH HHS/United States
- T32EB001680/EB/NIBIB NIH HHS/United States
- R01 NS052585/NS/NINDS NIH HHS/United States
- T32 EB001680/EB/NIBIB NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
