Anti-PrP Mab 6D11 suppresses PrP(Sc) replication in prion infected myeloid precursor line FDC-P1/22L and in the lymphoreticular system in vivo
- PMID: 19385058
- PMCID: PMC2713020
- DOI: 10.1016/j.nbd.2009.01.013
Anti-PrP Mab 6D11 suppresses PrP(Sc) replication in prion infected myeloid precursor line FDC-P1/22L and in the lymphoreticular system in vivo
Abstract
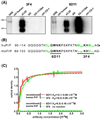
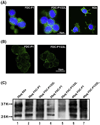
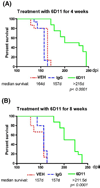
The pathogenesis of prion diseases is related to conformational transformation of cellular prion protein (PrP(C)) into a toxic, infectious, and self-replicating conformer termed PrP(Sc). Following extracerebral inoculation, the replication of PrP(Sc) is confined for months to years to the lymporeticular system (LRS) before the secondary CNS involvement results in occurrence of neurological symptoms. Therefore, replication of PrP(Sc), in the early stage of infection can be targeted by therapeutic approaches, which like passive immunization have limited blood-brain-barrier penetration. In this study, we show that 6D11 anti-PrP monoclonal antibody (Mab) prevents infection on a FDC-P1 myeloid precursor cell line stably infected with 22L mouse adapted scrapie strain. Passive immunization of extracerebrally infected CD-1 mice with Mab 6D11 resulted in effective suppression of PrP(Sc) replication in the LRS. Although, a rebound of PrP(Sc) presence occurred when the Mab 6D11 treatment was stopped, passively immunized mice showed a prolongation of the incubation period by 36.9% (pb0.0001) and a significant decrease in CNS pathology compared to control groups receiving vehicle or murine IgG. Our results indicate that antibody-based therapeutic strategies can be used, even on a short-term basis, to delay or prevent disease in subjects accidentally exposed to prions.
Figures



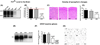

Similar articles
-
Anti-prion Protein Antibody 6D11 Restores Cellular Proteostasis of Prion Protein Through Disrupting Recycling Propagation of PrPSc and Targeting PrPSc for Lysosomal Degradation.Mol Neurobiol. 2019 Mar;56(3):2073-2091. doi: 10.1007/s12035-018-1208-4. Epub 2018 Jul 9. Mol Neurobiol. 2019. PMID: 29987703 Free PMC article.
-
Monoclonal antibodies inhibit prion replication and delay the development of prion disease.Nature. 2003 Mar 6;422(6927):80-3. doi: 10.1038/nature01457. Nature. 2003. PMID: 12621436
-
Clearance and prevention of prion infection in cell culture by anti-PrP antibodies.Eur J Neurosci. 2006 May;23(10):2635-47. doi: 10.1111/j.1460-9568.2006.04805.x. Eur J Neurosci. 2006. PMID: 16817866 Free PMC article.
-
Update on Creutzfeldt-Jakob disease.Curr Opin Neurol. 2004 Dec;17(6):641-7. doi: 10.1097/00019052-200412000-00002. Curr Opin Neurol. 2004. PMID: 15542971 Review.
-
In silico strategies on prion pathogenic conversion and inhibition from PrPC -PrPSc.Expert Opin Drug Discov. 2017 Mar;12(3):241-248. doi: 10.1080/17460441.2017.1287171. Epub 2017 Feb 2. Expert Opin Drug Discov. 2017. PMID: 28118747 Review.
Cited by
-
Immunomodulation for prion and prion-related diseases.Expert Rev Vaccines. 2010 Dec;9(12):1441-52. doi: 10.1586/erv.10.131. Expert Rev Vaccines. 2010. PMID: 21105779 Free PMC article. Review.
-
In vitro neutralization of prions with PrP(Sc)-specific antibodies.Prion. 2015;9(4):292-303. doi: 10.1080/19336896.2015.1071761. Prion. 2015. PMID: 26284508 Free PMC article.
-
Anti-PrPC monoclonal antibody infusion as a novel treatment for cognitive deficits in an Alzheimer's disease model mouse.BMC Neurosci. 2010 Oct 14;11:130. doi: 10.1186/1471-2202-11-130. BMC Neurosci. 2010. PMID: 20946660 Free PMC article.
-
Anti-prion Protein Antibody 6D11 Restores Cellular Proteostasis of Prion Protein Through Disrupting Recycling Propagation of PrPSc and Targeting PrPSc for Lysosomal Degradation.Mol Neurobiol. 2019 Mar;56(3):2073-2091. doi: 10.1007/s12035-018-1208-4. Epub 2018 Jul 9. Mol Neurobiol. 2019. PMID: 29987703 Free PMC article.
-
Passive Immunization With a Novel Monoclonal Anti-PrP Antibody TW1 in an Alzheimer's Mouse Model With Tau Pathology.Front Aging Neurosci. 2021 Feb 25;13:640677. doi: 10.3389/fnagi.2021.640677. eCollection 2021. Front Aging Neurosci. 2021. PMID: 33716717 Free PMC article.
References
-
- Aucouturier P, Frangione B, Wisniewski T. Prion diseases and the immune system. Ann. Med. Interne. 1999;150:75–78. - PubMed
-
- Beekes M, McBride PA. Early accumulation of pathological PrP in the enteric nervous system and gut-associated lymphoid tissue of hamsters orally infected with scrapie. Neurosci. Lett. 2000;278:181–184. - PubMed
-
- Beekes M, McBride PA, Baldauf E. Cerebral targeting indicates vagal spread of infection in hamsters fed with scrapie. J. Gen. Virol. 1998;3:601–607. - PubMed
-
- Bourette RP, Myles GM, Carlberg K, Chen AR, Rohrschneider LR. Uncoupling of the proliferation and differentiation signals mediated by the murine macrophage colony-stimulating factor receptor expressed in myeloid FDC-P1 cells. Cell Growth Differ. 1995;6:631–645. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
