From rapid place learning to behavioral performance: a key role for the intermediate hippocampus
- PMID: 19385719
- PMCID: PMC2671558
- DOI: 10.1371/journal.pbio.1000089
From rapid place learning to behavioral performance: a key role for the intermediate hippocampus
Abstract
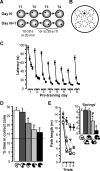
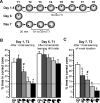
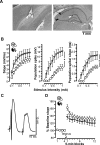
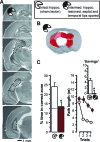
Rapid place encoding by hippocampal neurons, as reflected by place-related firing, has been intensely studied, whereas the substrates that translate hippocampal place codes into behavior have received little attention. A key point relevant to this translation is that hippocampal organization is characterized by functional-anatomical gradients along the septotemporal axis: Whereas the ability of hippocampal neurons to encode accurate place information declines from the septal to temporal end, hippocampal connectivity to prefrontal and subcortical sites that might relate such place information to behavioral-control processes shows an opposite gradient. We examined in rats the impact of selective lesions to relevant parts of the hippocampus on behavioral tests requiring place learning (watermaze procedures) and on in vivo electrophysiological models of hippocampal encoding (long-term potentiation [LTP], place cells). We found that the intermediate hippocampus is necessary and largely sufficient for behavioral performance based on rapid place learning. In contrast, a residual septal pole of the hippocampus, although displaying intact electrophysiological indices of rapid information encoding (LTP, precise place-related firing, and rapid remapping), failed to sustain watermaze performance based on rapid place learning. These data highlight the important distinction between hippocampal encoding and the behavioral performance based on such encoding, and suggest that the intermediate hippocampus, where substrates of rapid accurate place encoding converge with links to behavioral control, is critical to translate rapid (one-trial) place learning into navigational performance.
Conflict of interest statement
Competing interests. The authors have declared that no competing interests exist.
Figures


References
-
- Mackintosh NJ. Conditioning and associative learning. Oxford (United Kingdom): Clarendon Press; 1983. 316
-
- Tolman EC. Purposive behavior in animals and men. Berkeley (California): University of California Press; 1932. 463
-
- Cahill L, McGaugh JL, Weinberger NM. The neurobiology of learning and memory: some reminders to remember. Trends Neurosci. 2001;24:578–581. - PubMed
-
- Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav Brain Sci. 1999;22:425–444. - PubMed
-
- Burgess N, Maguire EA, O'Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–641. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

