The effect of varying analytical methods on estimates of anti-malarial clinical efficacy
- PMID: 19386088
- PMCID: PMC2679050
- DOI: 10.1186/1475-2875-8-77
The effect of varying analytical methods on estimates of anti-malarial clinical efficacy
Abstract
Background: Analytical approaches for the interpretation of anti-malarial clinical trials vary considerably. The aim of this study was to quantify the magnitude of the differences between efficacy estimates derived from these approaches and identify the factors underlying these differences.
Methods: Data from studies conducted in Africa and Thailand were compiled and the risk estimates of treatment failure, adjusted and unadjusted by genotyping, were derived by three methods (intention to treat (ITT), modified intention to treat (mITT) and per protocol (PP)) and then compared.
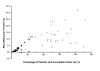
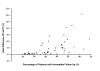
Results: 29 clinical trials (15 from Africa and 14 from Thailand) with a total of 65 treatment arms (38 from Africa and 27 from Thailand) were included in the analysis. Of the 15,409 patients enrolled, 2,637 (17.1%) had incomplete follow up for the unadjusted analysis and 4,489 (33.4%) for the adjusted analysis. Estimates of treatment failure were consistently higher when derived from the ITT or PP analyses compared to the mITT approach. In the unadjusted analyses the median difference between the ITT and mITT estimates was greater in Thai studies (11.4% [range 2.1-31.8]) compared to African Studies (1.8% [range 0-11.7]). In the adjusted analyses the median difference between PP and mITT estimates was 1.7%, but ranged from 0 to 30.9%. The discrepancy between estimates was correlated significantly with the proportion of patients with incomplete follow-up; p < 0.0001. The proportion of studies with a major difference (> 5%) between adjusted PP and mITT was 28% (16/57), with the risk difference greater in African (37% 14/38) compared to Thai studies (11% 2/19). In the African studies, a major difference in the adjusted estimates was significantly more likely in studies in high transmission sites (62% 8/13) compared to studies in moderate transmission sites (24% 6/25); p = 0.035.
Conclusion: Estimates of anti-malarial clinical efficacy vary significantly depending on the analytical methodology from which they are derived. In order to monitor temporal and spatial trends in anti-malarial efficacy, standardized analytical tools need to be applied in a transparent and systematic manner.
Figures
Similar articles
-
Statistical methods to derive efficacy estimates of anti-malarials for uncomplicated Plasmodium falciparum malaria: pitfalls and challenges.Malar J. 2017 Oct 26;16(1):430. doi: 10.1186/s12936-017-2074-7. Malar J. 2017. PMID: 29073901 Free PMC article. Review.
-
Intervals to Plasmodium falciparum recurrence after anti-malarial treatment in pregnancy: a longitudinal prospective cohort.Malar J. 2015 May 28;14:221. doi: 10.1186/s12936-015-0745-9. Malar J. 2015. PMID: 26017553 Free PMC article.
-
Some considerations in the design and interpretation of antimalarial drug trials in uncomplicated falciparum malaria.Malar J. 2006 Dec 22;5:127. doi: 10.1186/1475-2875-5-127. Malar J. 2006. PMID: 17187673 Free PMC article. Review.
-
Randomized, multicentre assessment of the efficacy and safety of ASAQ--a fixed-dose artesunate-amodiaquine combination therapy in the treatment of uncomplicated Plasmodium falciparum malaria.Malar J. 2009 Jun 8;8:125. doi: 10.1186/1475-2875-8-125. Malar J. 2009. PMID: 19505304 Free PMC article. Clinical Trial.
-
Different methodological approaches to the assessment of in vivo efficacy of three artemisinin-based combination antimalarial treatments for the treatment of uncomplicated falciparum malaria in African children.Malar J. 2008 Aug 9;7:154. doi: 10.1186/1475-2875-7-154. Malar J. 2008. PMID: 18691429 Free PMC article.
Cited by
-
Associations between antibodies to a panel of Plasmodium falciparum specific antigens and response to sub-optimal antimalarial therapy in Kampala, Uganda.PLoS One. 2012;7(12):e52571. doi: 10.1371/journal.pone.0052571. Epub 2012 Dec 19. PLoS One. 2012. PMID: 23285095 Free PMC article. Clinical Trial.
-
Monitoring antimalarial drug resistance: Applying lessons learned from the past in a fast-moving present.Int J Parasitol Drugs Drug Resist. 2012 Apr 20;2:126-33. doi: 10.1016/j.ijpddr.2012.03.004. eCollection 2012 Dec. Int J Parasitol Drugs Drug Resist. 2012. PMID: 24533274 Free PMC article. Review.
-
Efficacy of fixed-dose combination artesunate-amodiaquine versus artemether-lumefantrine for uncomplicated childhood Plasmodium falciparum malaria in Democratic Republic of Congo: a randomized non-inferiority trial.Malar J. 2012 May 25;11:174. doi: 10.1186/1475-2875-11-174. Malar J. 2012. PMID: 22631564 Free PMC article. Clinical Trial.
-
A Phase 3, Double-Blind, Randomized Study of Arterolane Maleate-Piperaquine Phosphate vs Artemether-Lumefantrine for Falciparum Malaria in Adolescent and Adult Patients in Asia and Africa.Clin Infect Dis. 2016 Apr 15;62(8):964-971. doi: 10.1093/cid/ciw029. Epub 2016 Feb 21. Clin Infect Dis. 2016. PMID: 26908796 Free PMC article. Clinical Trial.
-
Statistical methods to derive efficacy estimates of anti-malarials for uncomplicated Plasmodium falciparum malaria: pitfalls and challenges.Malar J. 2017 Oct 26;16(1):430. doi: 10.1186/s12936-017-2074-7. Malar J. 2017. PMID: 29073901 Free PMC article. Review.
References
-
- WHO Guidelines for the treatment of malaria. Document No WHO/HTM/MAL/20061108.
-
- Guthmann JP, Pinoges L, Checchi F, Cousens S, Balkan S, van Herp M, Legros D, Olliaro P. Methodological issues in the assessment of antimalarial drug treatment: analysis of 13 studies in eight African countries from 2001 to 2004. Antimicrob Agents Chemother. 2006;50:3734–3739. doi: 10.1128/AAC.01618-05. - DOI - PMC - PubMed
-
- Ashley EA, Pinoges L, Turyakira E, Dorsey G, Checchi F, Bukirwa H, Broek I van den, Zongo I, Urruta PP, van Herp M, Balkan S, Taylor WR, Olliaro P, Guthmann JP. Different methodological approaches to the assessment of in vivo efficacy of three artemisinin-based combination antimalarial treatments for the treatment of uncomplicated falciparum malaria in African children. Malar J. 2008;7:154. doi: 10.1186/1475-2875-7-154. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

