Pregnancy in the mature adult mouse does not alter the proportion of mammary epithelial stem/progenitor cells
- PMID: 19386118
- PMCID: PMC2688949
- DOI: 10.1186/bcr2245
Pregnancy in the mature adult mouse does not alter the proportion of mammary epithelial stem/progenitor cells
Abstract
Introduction: In humans, an early full-term pregnancy reduces lifetime breast cancer risk by up to 50% whereas a later pregnancy (>35 years old) can increase lifetime risk. Several mechanisms have been suggested, including changes in levels of circulating hormones, changes in the way the breast responds to these hormones, changes in gene expression programmes which may alter susceptibility to transformation and changes to mammary stem cell numbers or behaviour. Previous studies have shown that the mammary tissue isolated from both virgin and parous mice has the ability to repopulate a cleared mammary fat pad in transplant experiments. Limited dilution transplant assays have demonstrated that early pregnancy (at 5 weeks of age) reduces stem/progenitor cell numbers in the mouse mammary epithelium by twofold. However, the effects on stem/progenitor cell numbers in the mammary epithelium of a pregnancy in older animals have not yet been tested.
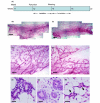
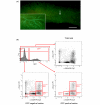
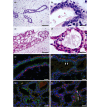
Methods: Mice were put through a full-term pregnancy at 9 weeks of age, when the mammary epithelium is mature. The total mammary epithelium was purified from parous 7-week post-lactation and age-matched virgin mice and analysed by flow cytometry and limiting dilution cleared fat pad transplants.
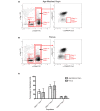
Results: There were no significant differences in the proportions of different mammary epithelial cell populations or numbers of CD24(+/Low) Sca-1- CD49f(High) cells (stem cell enriched basal mammary epithelial compartment). There was no significant difference in stem/progenitor cell frequency based on limiting dilution transplants between the parous and age-matched virgin epithelium.
Conclusions: Although differences between parous and virgin mammary epithelium at later time points post lactation or following multiple pregnancies cannot be ruled out, there are no differences in stem/progenitor cell numbers between mammary epithelium isolated from parous animals which were mated at 9 weeks old and virgin animals. However, a recent report has suggested that animals that were mated at 5 weeks old have a twofold reduction in stem/progenitor cell numbers. This is of interest given the association between early, but not late, pregnancy and breast cancer risk reduction in humans. However, a mechanistic connection between stem cell numbers and breast cancer risk remains to be established.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

