Plk1-dependent and -independent roles of an ODF2 splice variant, hCenexin1, at the centrosome of somatic cells
- PMID: 19386263
- PMCID: PMC2741019
- DOI: 10.1016/j.devcel.2009.02.004
Plk1-dependent and -independent roles of an ODF2 splice variant, hCenexin1, at the centrosome of somatic cells
Abstract
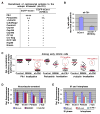
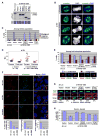
Outer dense fiber 2 (ODF2) was initially identified as a major component of the sperm tail cytoskeleton, and was later suggested to be localized to somatic centrosomes and required for the formation of primary cilia. Here we show that a splice variant of hODF2 called hCenexin1, but not hODF2 itself, efficiently localizes to somatic centrosomes via a variant-specific C-terminal extension and recruits Plk1 through a Cdc2-dependent phospho-S796 motif within the extension. This interaction and Plk1 activity were important for proper recruitment of pericentrin and gamma-tubulin, and, ultimately, for formation of normal bipolar spindles. Earlier in the cell cycle, hCenexin1, but again not hODF2, also contributed to centrosomal recruitment of ninein and primary cilia formation independent of Plk1 interaction. These findings provide a striking example of how a splice-generated C-terminal extension of a sperm tail-associating protein mediates unanticipated centrosomal events at distinct stages of the somatic cell cycle.
Figures
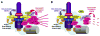
References
-
- Barr FA, Sillje HH, Nigg EA. Polo-like kinases and the orchestration of cell division. Nat Rev Mol Cell Biol. 2004;5:429–440. - PubMed
-
- Bellanger S, de Gramont A, Sobczak-Thépot J. Cyclin B2 suppresses mitotic failure and DNA re-replication in human somatic cells knocked down for both cyclins B1 and B2. Oncogene. 2007;26:7175–7184. - PubMed
-
- Brohmann H, Pinnecke S, Hoyer-Fender S. Identification and characterization of new cDNAs encoding outer dense fiber proteins of rat sperm. J Biol Chem. 1997;272:10327–10332. - PubMed
-
- Casenghi M, Meraldi P, Weinhart U, Duncan PI, Korner R, Nigg EA. Polo-like kinase 1 regulates Nlp, a centrosome protein involved in microtubule nucleation. Dev Cell. 2003;5:113–125. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

