Linking asymmetric cell division to the terminal differentiation program of postmitotic neurons in C. elegans
- PMID: 19386265
- PMCID: PMC2691723
- DOI: 10.1016/j.devcel.2009.02.011
Linking asymmetric cell division to the terminal differentiation program of postmitotic neurons in C. elegans
Abstract
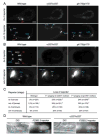
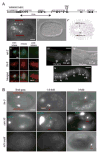
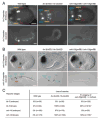
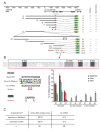
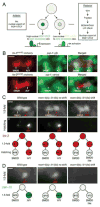
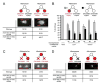
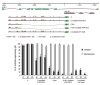
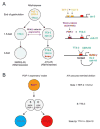
How asymmetric divisions are connected to the terminal differentiation program of neuronal subtypes is poorly understood. In C. elegans, two homeodomain transcription factors, TTX-3 (a LHX2/9 ortholog) and CEH-10 (a CHX10 ortholog), directly activate a large battery of terminal differentiation genes in the cholinergic interneuron AIY. We establish here a transcriptional cascade linking asymmetric division to this differentiation program. A transient lineage-specific input formed by the Zic factor REF-2 and the bHLH factor HLH-2 directly activates ttx-3 expression in the AIY mother. During the terminal division of the AIY mother, an asymmetric Wnt/beta-catenin pathway cooperates with TTX-3 to directly restrict ceh-10 expression to only one of the two daughter cells. TTX-3 and CEH-10 automaintain their expression, thereby locking in the differentiation state. Our study establishes how transient lineage and asymmetric division inputs are integrated and suggests that the Wnt/beta-catenin pathway is widely used to control the identity of neuronal lineages.
Figures
Similar articles
-
Wnt ligands regulate the asymmetric divisions of neuronal progenitors in C. elegans embryos.Development. 2020 Apr 6;147(7):dev183186. doi: 10.1242/dev.183186. Development. 2020. PMID: 32156756 Free PMC article.
-
A regulatory cascade of three homeobox genes, ceh-10, ttx-3 and ceh-23, controls cell fate specification of a defined interneuron class in C. elegans.Development. 2001 Jun;128(11):1951-69. doi: 10.1242/dev.128.11.1951. Development. 2001. PMID: 11493519
-
Otx-dependent expression of proneural bHLH genes establishes a neuronal bilateral asymmetry in C. elegans.Development. 2010 Dec;137(23):4017-27. doi: 10.1242/dev.058834. Epub 2010 Nov 1. Development. 2010. PMID: 21041366 Free PMC article.
-
Two betas or not two betas: regulation of asymmetric division by beta-catenin.Trends Cell Biol. 2007 Oct;17(10):465-73. doi: 10.1016/j.tcb.2007.08.004. Epub 2007 Oct 4. Trends Cell Biol. 2007. PMID: 17919911 Review.
-
Wnt Signaling Polarizes C. elegans Asymmetric Cell Divisions During Development.Results Probl Cell Differ. 2017;61:83-114. doi: 10.1007/978-3-319-53150-2_4. Results Probl Cell Differ. 2017. PMID: 28409301 Free PMC article. Review.
Cited by
-
Integration of Spatial and Temporal Patterning in the Invertebrate and Vertebrate Nervous System.Front Neurosci. 2022 Mar 22;16:854422. doi: 10.3389/fnins.2022.854422. eCollection 2022. Front Neurosci. 2022. PMID: 35392413 Free PMC article. Review.
-
Open Frontiers in Neural Cell Type Investigations; Lessons From Caenorhabditis elegans and Beyond, Toward a Multimodal Integration.Front Neurosci. 2022 Mar 7;15:787753. doi: 10.3389/fnins.2021.787753. eCollection 2021. Front Neurosci. 2022. PMID: 35321480 Free PMC article. Review.
-
A map of terminal regulators of neuronal identity in Caenorhabditis elegans.Wiley Interdiscip Rev Dev Biol. 2016 Jul;5(4):474-98. doi: 10.1002/wdev.233. Epub 2016 May 2. Wiley Interdiscip Rev Dev Biol. 2016. PMID: 27136279 Free PMC article. Review.
-
Deep learning-based aberration compensation improves contrast and resolution in fluorescence microscopy.Nat Commun. 2025 Jan 2;16(1):313. doi: 10.1038/s41467-024-55267-x. Nat Commun. 2025. PMID: 39747824 Free PMC article.
-
Maintenance of postmitotic neuronal cell identity.Nat Neurosci. 2014 Jul;17(7):899-907. doi: 10.1038/nn.3731. Epub 2014 Jun 15. Nat Neurosci. 2014. PMID: 24929660 Free PMC article. Review.
References
-
- Alper S, Kenyon C. The zinc finger protein REF-2 functions with the Hox genes to inhibit cell fusion in the ventral epidermis of C. elegans. Development. 2002;129:3335–3348. - PubMed
-
- Altun-Gultekin Z, Andachi Y, Tsalik EL, Pilgrim D, Kohara Y, Hobert O. A regulatory cascade of three homeobox genes, ceh-10, ttx-3 and ceh-23, controls cell fate specification of a defined interneuron class in C. elegans. Development. 2001;128:1951–1969. - PubMed
-
- Arata Y, Kouike H, Zhang Y, Herman MA, Okano H, Sawa H. Wnt signaling and a Hox protein cooperatively regulate psa-3/Meis to determine daughter cell fate after asymmetric cell division in C. elegans. Dev Cell. 2006;11:105–115. - PubMed
-
- Aruga J. The role of Zic genes in neural development. Mol Cell Neurosci. 2004;26:205–221. - PubMed
-
- Asbreuk CH, van Schaick HS, Cox JJ, Kromkamp M, Smidt MP, Burbach JP. The homeobox genes Lhx7 and Gbx1 are expressed in the basal forebrain cholinergic system. Neuroscience. 2002;109:287–298. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

