Meningioma 1 is required for appropriate osteoblast proliferation, motility, differentiation, and function
- PMID: 19386590
- PMCID: PMC2709374
- DOI: 10.1074/jbc.M109.001354
Meningioma 1 is required for appropriate osteoblast proliferation, motility, differentiation, and function
Abstract
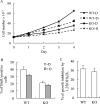
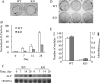
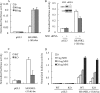
The vitamin D endocrine system is essential for calcium and phosphate homeostasis and skeletal mineralization. The 1,25-dihydroxyvitamin D(3) (1,25(OH)(2)D(3)) hormone binds to the vitamin D receptor (VDR) to regulate gene expression. These gene products in turn mediate the actions of 1,25(OH)(2)D(3) in mineral-regulating target cells such as the osteoblast. We showed previously that meningioma 1 (MN1) is a novel target of 1,25(OH)(2)D(3) in MG-63 osteoblastic cells and that it is a coactivator for VDR-mediated transcription (Sutton, A. L., Zhang, X., Ellison, T. I., and MacDonald, P. N. (2005) Mol. Endocrinol. 19, 2234-2244). However, the functional significance of MN1 in osteoblastic cell biology is largely unknown. Here, we demonstrate that MN1 expression is increased dramatically during differentiation of primary osteoblastic cells. Using calvarial osteoblasts derived from wild-type and MN1 knock-out mice, we provide data supporting an essential role of MN1 in maintaining appropriate osteoblast proliferation, differentiation, and function. MN1 knock-out osteoblasts displayed altered morphology, decreased growth rate, impaired motility, and attenuated 1,25(OH)(2)D(3)/VDR-mediated transcription as well as reduced alkaline phosphatase activity and mineralized nodule formation. MN1 null osteoblasts were also impaired in supporting osteoclastogenesis in co-culture studies presumably because of marked reduction in the RANKL:OPG ratio in the MN1 null cells. Mechanistic studies supported a transcriptional role for MN1 in controlling RANKL gene expression through activation of the RANKL promoter. Cumulatively, these studies indicate an important role for MN1 in maintaining the appropriate maturation and function of calvarial osteoblasts.
Figures

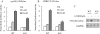


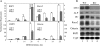
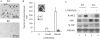
References
-
- Bianco P., Riminucci M., Gronthos S., Robey P. G. ( 2001) Stem Cells 19, 180– 192 - PubMed
-
- Kassem M., Abdallah B. M., Saeed H. ( 2008) Arch. Biochem. Biophys. 473, 183– 187 - PubMed
-
- Aubin J. E. ( 1998) J. Cell. Biochem. Suppl. 30–31, 73– 82 - PubMed
-
- Miyamoto T., Suda T. ( 2003) Keio J. Med. 52, 1– 7 - PubMed
-
- Teitelbaum S. L., Ross F. P. ( 2003) Nat. Rev. Genet. 4, 638– 649 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

