Decreased TCF7L2 protein levels in type 2 diabetes mellitus correlate with downregulation of GIP- and GLP-1 receptors and impaired beta-cell function
- PMID: 19386626
- PMCID: PMC2722186
- DOI: 10.1093/hmg/ddp178
Decreased TCF7L2 protein levels in type 2 diabetes mellitus correlate with downregulation of GIP- and GLP-1 receptors and impaired beta-cell function
Erratum in
-
Decreased TCF7L2 protein levels in type 2 diabetes mellitus correlate with downregulation of GIP- and GLP-1 receptors and impaired beta-cell function.Hum Mol Genet. 2015 May 15;24(10):3004. doi: 10.1093/hmg/ddv075. Epub 2015 Mar 8. Hum Mol Genet. 2015. PMID: 25753258 Free PMC article.
Abstract
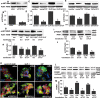
Recent human genetics studies have revealed that common variants of the TCF7L2 (T-cell factor 7-like 2, formerly known as TCF4) gene are strongly associated with type 2 diabetes mellitus (T2DM). We have shown that TCF7L2 expression in the beta-cells is correlated with function and survival of the insulin-producing pancreatic beta-cell. In order to understand how variations in TCF7L2 influence diabetes progression, we investigated its mechanism of action in the beta-cell. We show robust differences in TCF7L2 expression between healthy controls and models of T2DM. While mRNA levels were approximately 2-fold increased in isolated islets from the diabetic db/db mouse, the Vancouver Diabetic Fatty (VDF) Zucker rat and the high fat/high sucrose diet-treated mouse compared with the non-diabetic controls, protein levels were decreased. A similar decrease was observed in pancreatic sections from patients with T2DM. In parallel, expression of the receptors for glucagon-like peptide 1 (GLP-1R) and glucose-dependent insulinotropic polypeptide (GIP-R) was decreased in islets from humans with T2DM as well as in isolated human islets treated with siRNA to TCF7L2 (siTCF7L2). Also, insulin secretion stimulated by glucose, GLP-1 and GIP, but not KCl or cyclic adenosine monophosphate (cAMP) was impaired in siTCF7L2-treated isolated human islets. Loss of TCF7L2 resulted in decreased GLP-1 and GIP-stimulated AKT phosphorylation, and AKT-mediated Foxo-1 phosphorylation and nuclear exclusion. Our findings suggest that beta-cell function and survival are regulated through an interplay between TCF7L2 and GLP-1R/GIP-R expression and signaling in T2DM.
Figures



References
-
- Florez J.C. The new type 2 diabetes gene TCF7L2. Curr. Opin. Clin. Nutr. Metab. Care. 2007;10:391–396. - PubMed
-
- Sladek R., Rocheleau G., Rung J., Dina C., Shen L., Serre D., Boutin P., Vincent D., Belisle A., Hadjadj S., et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881–885. - PubMed
-
- Nauck M.A., Meier J.J. The enteroinsular axis may mediate the diabetogenic effects of TCF7L2 polymorphisms. Diabetologia. 2007;50:2413–2416. - PubMed
-
- Schafer S.A., Tschritter O., Machicao F., Thamer C., Stefan N., Gallwitz B., Holst J.J., Dekker J.M., t'Hart L.M., Nijpels G., et al. Impaired glucagon-like peptide-1-induced insulin secretion in carriers of transcription factor 7-like 2 (TCF7L2) gene polymorphisms. Diabetologia. 2007;50:2443–2450. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

