Human papillomavirus type 16 (HPV-16) virus-like particle L1-specific CD8+ cytotoxic T lymphocytes (CTLs) are equally effective as E7-specific CD8+ CTLs in killing autologous HPV-16-positive tumor cells in cervical cancer patients: implications for L1 dendritic cell-based therapeutic vaccines
- PMID: 19386711
- PMCID: PMC2698533
- DOI: 10.1128/JVI.02443-08
Human papillomavirus type 16 (HPV-16) virus-like particle L1-specific CD8+ cytotoxic T lymphocytes (CTLs) are equally effective as E7-specific CD8+ CTLs in killing autologous HPV-16-positive tumor cells in cervical cancer patients: implications for L1 dendritic cell-based therapeutic vaccines
Abstract
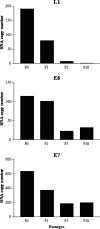
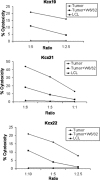
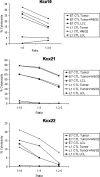
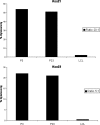
Papillomavirus-like particles (VLPs) based on L1 capsid protein represent a promising prophylactic vaccine against human papillomavirus (HPV) infections. However, cell-mediated immune responses against this antigen are believed to be of limited therapeutic value in established HPV-infected cervical lesions and, for this reason, have not been intensively investigated in cervical cancer patients. In this study we analyzed and quantified by real-time PCR (RT-PCR) the RNA expression levels of E6, E7, and L1 genes in flash-frozen HPV-16 cervical carcinomas. In addition, the kinetics of expression of E6, E7, and L1 in HPV-16-infected primary cell lines established as long-term cultures in vitro was also evaluated at RNA and protein levels. Finally, in order to evaluate the therapeutic potential of L1-specific CD4(+) and CD8(+) T lymphocytes responses in cervical cancer patients, L1 VLP-loaded dendritic cells (DCs) were used to stimulate peripheral blood lymphocytes from cervical cancer patients and such responses were compared to those elicited by the E7 oncoprotein. We show that 22 of 22 (100%) flash-frozen cervical biopsy samples collected from HPV-16-positive cervical cancer patients harbor L1, in addition to E6 and E7 RNA, as detected by RT-PCR. E7 RNA copy number (mean, 176.2) was significantly higher in HPV-16-positive cervical cancers compared to the E6 RNA copy number (mean, 47.3) and the L1 copy number (mean, 58.3) (P < 0.0001 and P < 0.001, respectively). However, no significant differences in expression levels between E6 and L1 were found. Kinetic studies of E6, E7, and L1 RNA and protein expression levels in primary tumors showed a sharp reduction in L1 expression after multiple in vitro passages compared to E6 and E7. Autologous DCs pulsed with HPV-16 VLPs or recombinant full-length E7 elicited strong type 1 L1- and E7-specific responses in CD4(+) and CD8(+) T cells from cervical cancer patients. Importantly, L1 VLP-specific CD8(+) T lymphocytes expressed strong cytolytic activity against autologous tumor cells and were as effective as E7-specific cytotoxic T lymphocytes in lysing naturally HPV-16-infected autologous tumor cells. Taken together, these data demonstrate a consistent expression of L1 in primary cervical tumors and the possibility of inducing effective L1/tumor-specific CD4(+) and CD8(+) T-lymphocyte responses in patients harboring HPV-infected cervical cancer. These results may have important implications for the treatment of patients harboring established HPV-infected lesions with L1 VLPs or combined E7/L1 DC-based vaccinations.
Figures


References
-
- Aiba, S., M. Rokugo, and H. Tagami. 1986. Immunohistologic analysis of the phenomenon of spontaneous regression of numerous flat warts. Cancer 581246-1251. - PubMed
-
- Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392245-252. - PubMed
-
- Coleman, N., H. D. Birley, A. M. Renton, N. F. Hanna, B. K. Ryait, M. Byrne, D. Taylor-Robinson, and M. A. Stanley. 1994. Immunological events in regressing genital warts. Am. J. Clin. Pathol. 102768-774. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

