Expression and modulation of translocator protein and its partners by hypoxia reoxygenation or ischemia and reperfusion in porcine renal models
- PMID: 19386723
- PMCID: PMC2711711
- DOI: 10.1152/ajprenal.90422.2008
Expression and modulation of translocator protein and its partners by hypoxia reoxygenation or ischemia and reperfusion in porcine renal models
Abstract
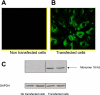
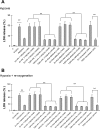
Translocator protein (TSPO), formerly known as the peripheral-type benzodiazepine receptor, is an 18-kDa drug- and cholesterol-binding protein localized to the outer mitochondrial membrane and implicated in a variety of cell and mitochondrial functions. To determine the role of TSPO in ischemia-reperfusion injury (IRI), we used both in vivo and in vitro porcine models: an in vivo renal ischemia model where different conservation modalities were tested and an in vitro model where TSPO-transfected porcine proximal tubule LLC-PK(1) cells were exposed to hypoxia and oxidative stress. The expression of TSPO and its partners in steroidogenic cells, steroidogenic acute regulatory protein (StAR) and cytochrome P-450 side chain cleavage CYP11A1, as well as the impact of TSPO overexpression and exposure to TSPO ligands in vitro in hypoxia-ischemia conditions were investigated. Hypoxia induced caspase activation, reduction of ATP content, and LLC-PK(1) cell death. Transfection and overexpression of TSPO rescued the cells from the detrimental effects of hypoxia and reoxygenation. Moreover, TSPO overexpression was accompanied by a reduction of H(2)O(2)-induced necrosis. TSPO drug ligands did not affect TSPO-mediated functions. In vivo, TSPO expression was modulated by IRI and during regeneration particularly in proximal tubule cells, which do not express this protein at the basal level. Under the same conditions, StAR and CYP11A1 protein and gene expression was reduced without apparent relation to TSPO changes. Pregnenolone was identified and measured in the pig kidney. Pregnenolone synthesis was not affected by the experimental conditions used. Taken together, these results indicate that changes in TSPO expression in kidney regenerating tissue could be important for renal protection and maintenance of kidney function.
Figures








Similar articles
-
Role of translocator protein in renal ischemia reperfusion, renal preservation and acute kidney injury.Curr Mol Med. 2012 May;12(4):413-25. Curr Mol Med. 2012. PMID: 22364128 Review.
-
Steroid hormone synthesis in mitochondria.Mol Cell Endocrinol. 2013 Oct 15;379(1-2):62-73. doi: 10.1016/j.mce.2013.04.014. Epub 2013 Apr 28. Mol Cell Endocrinol. 2013. PMID: 23628605
-
Expression of the RNA-stabilizing protein HuR in ischemia-reperfusion injury of rat kidney.Am J Physiol Renal Physiol. 2009 Jul;297(1):F95-F105. doi: 10.1152/ajprenal.90632.2008. Epub 2009 May 6. Am J Physiol Renal Physiol. 2009. PMID: 19420108 Free PMC article.
-
Localization and functional activity of cytochrome P450 side chain cleavage enzyme (CYP11A1) in the adult rat kidney.Mol Cell Endocrinol. 2011 Jan 30;332(1-2):253-60. doi: 10.1016/j.mce.2010.10.020. Epub 2010 Nov 12. Mol Cell Endocrinol. 2011. PMID: 21075169
-
Role of apoptosis in hypoxic/ischemic damage in the kidney.Semin Nephrol. 2003 Nov;23(6):511-21. doi: 10.1053/s0270-9295(03)00130-x. Semin Nephrol. 2003. PMID: 14631559 Review.
Cited by
-
Gender difference and sex hormone production in rodent renal ischemia reperfusion injury and repair.J Inflamm (Lond). 2011 Jun 9;8:14. doi: 10.1186/1476-9255-8-14. J Inflamm (Lond). 2011. PMID: 21658244 Free PMC article.
-
Essential Principles and Recent Progress in the Development of TSPO PET Ligands for Neuroinflammation Imaging.Curr Med Chem. 2022 Aug 6;29(28):4862-4890. doi: 10.2174/0929867329666220329204054. Curr Med Chem. 2022. PMID: 35352645 Free PMC article. Review.
-
Animal Models of Kidney Disease: Challenges and Perspectives.Kidney360. 2023 Oct 1;4(10):1479-1493. doi: 10.34067/KID.0000000000000227. Kidney360. 2023. PMID: 37526653 Free PMC article. Review.
-
ABA, porphyrins and plant TSPO-related protein.Plant Signal Behav. 2009 Nov;4(11):1087-90. doi: 10.4161/psb.4.11.9796. Epub 2009 Nov 12. Plant Signal Behav. 2009. PMID: 19838071 Free PMC article.
-
Analysis of machine perfusion benefits in kidney grafts: a preclinical study.J Transl Med. 2011 Jan 25;9:15. doi: 10.1186/1479-5876-9-15. J Transl Med. 2011. PMID: 21266040 Free PMC article.
References
-
- Azarashvili T, Grachev D, Krestinina O, Evtodienko Y, Yurkov I, Papadopoulos V, Reiser G. The peripheral-type benzodiazepine receptor is involved in control of Ca2+-induced permeability transition pore opening in rat brain mitochondria. Cell Calcium 42: 27–39, 2007. - PubMed
-
- Benitez-Bribiesca L, Gomez-Camarillo M, Castellanos-Juarez E, Mravko E, Sanchez-Suarez P. Morphologic, biochemical and molecular mitochondrial changes during reperfusion phase following brief renal ischemia. Ann NY Acad Sci 926: 165–179, 2000. - PubMed
-
- Blomberg LA, Zuelke KA. Expression analysis of the steroidogenic acute regulatory protein (STAR) gene in developing porcine concept uses. Mol Reprod Dev 72: 419–429, 2005. - PubMed
-
- Bono F, Lamarche I, Prabonnaud V, Le Fur G, Herbert JM. Peripheral benzodiazepine receptor agonists exhibit potent antiapoptotic activities. Biochem Biophys Res Commun 265: 457–461, 1999. - PubMed
-
- Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol 14: 2199–2210, 2003. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

