Task-dependent modulation of primary afferent depolarization in cervical spinal cord of monkeys performing an instructed delay task
- PMID: 19386753
- PMCID: PMC2712276
- DOI: 10.1152/jn.91113.2008
Task-dependent modulation of primary afferent depolarization in cervical spinal cord of monkeys performing an instructed delay task
Abstract
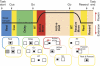
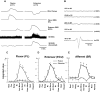
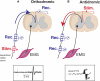
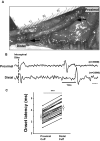
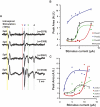
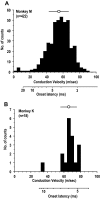
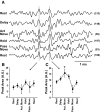
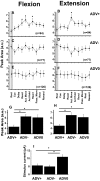
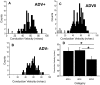
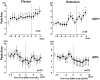
Task-dependent modulation of primary afferent depolarization (PAD) was studied in the cervical spinal cord of two monkeys performing a wrist flexion and extension task with an instructed delay period. We implanted two nerve cuff electrodes on proximal and distal parts of the superficial radial nerve (SR) and a recording chamber over a hemi-laminectomy in the lower cervical vertebrae. Antidromic volleys (ADVs) in the SR were evoked by intraspinal microstimuli (ISMS, 3-10 Hz, 3-30 microA) applied through a tungsten microelectrode, and the area of each ADV was measured. In total, 434 ADVs were evoked by ISMS in two monkeys, with onset latency consistently shorter in the proximal than distal cuffs. Estimated conduction velocity suggest that most ADVs were caused by action potentials in cutaneous fibers originating from low-threshold tactile receptors. Modulation of the size of ADVs as a function of the task was examined in 281 ADVs induced by ISMS applied at 78 different intraspinal sites. The ADVs were significantly facilitated during active movement in both flexion and extension (P<0.05), suggesting an epoch-dependent modulation of PAD. This facilitation started 400-900 ms before the onset of EMG activity. Such pre-EMG modulation is hard to explain by movement-induced reafference and probably is associated with descending motor commands.
Figures


References
-
- Andersen P, Eccles JC, Sears TA. Cortically evoked depolarization of primary afferent fibers in the spinal cord. J Neurophysiol 27: 63–77, 1964. - PubMed
-
- Baev KV, Panchin Iu V, Skryma RN. Depolarization of primary afferents during fictitious scratching of thalamic cats. Neirofiziologiia 10: 173–176, 1978. - PubMed
-
- Baldissera F, Broggi G, Mancia M. Primary afferent depolarization of trigeminal fibres induced by stimulation of brain stem and peripheral nerves. Experientia 23: 398–400, 1967. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

