Humanin inhibits neuronal cell death by interacting with a cytokine receptor complex or complexes involving CNTF receptor alpha/WSX-1/gp130
- PMID: 19386761
- PMCID: PMC2695794
- DOI: 10.1091/mbc.e09-02-0168
Humanin inhibits neuronal cell death by interacting with a cytokine receptor complex or complexes involving CNTF receptor alpha/WSX-1/gp130
Abstract
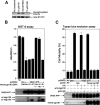
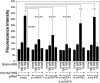
Humanin (HN) inhibits neuronal death induced by various Alzheimer's disease (AD)-related insults via an unknown receptor on cell membranes. Our earlier study indicated that the activation of STAT3 was essential for HN-induced neuroprotection, suggesting that the HN receptor may belong to the cytokine receptor family. In this study, a series of loss-of-function tests indicated that gp130, the common subunit of receptors belonging to the IL-6 receptor family, was essential for HN-induced neuroprotection. Overexpression of ciliary neurotrophic factor receptor alpha (CNTFR) and/or the IL-27 receptor subunit, WSX-1, but not that of any other tested gp130-related receptor subunit, up-regulated HN binding to neuronal cells, whereas siRNA-mediated knockdown of endogenous CNTFR and/or WSX-1 reduced it. These results suggest that both CNTFR and WSX-1 may be also involved in HN binding to cells. Consistent with these results, loss-of-functions of CNTFR or WSX-1 in neuronal cells nullified their responsiveness to HN-mediated protection. In vitro-reconstituted binding assays showed that HN, but not the other control peptide, induced the hetero-oligomerization of CNTFR, WSX-1, and gp130. Together, these results indicate that HN protects neurons by binding to a complex or complexes involving CNTFR/WSX-1/gp130.
Figures

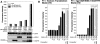



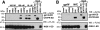
Similar articles
-
Identification of soluble WSX-1 not as a dominant-negative but as an alternative functional subunit of a receptor for an anti-Alzheimer's disease rescue factor Humanin.Biochem Biophys Res Commun. 2009 Nov 6;389(1):95-9. doi: 10.1016/j.bbrc.2009.08.095. Epub 2009 Aug 22. Biochem Biophys Res Commun. 2009. PMID: 19703422
-
The IL-27 component EBI-3 and its receptor subunit IL-27Rα are essential for the cytoprotective action of humanin on male germ cells†.Biol Reprod. 2021 Mar 11;104(3):717-730. doi: 10.1093/biolre/ioaa225. Biol Reprod. 2021. PMID: 33330922 Free PMC article.
-
The amino acid exchange R28E in ciliary neurotrophic factor (CNTF) abrogates interleukin-6 receptor-dependent but retains CNTF receptor-dependent signaling via glycoprotein 130 (gp130)/leukemia inhibitory factor receptor (LIFR).J Biol Chem. 2014 Jun 27;289(26):18442-50. doi: 10.1074/jbc.M114.568857. Epub 2014 May 6. J Biol Chem. 2014. PMID: 24802752 Free PMC article.
-
The ciliary neurotrophic factor and its receptor, CNTFR alpha.Pharm Acta Helv. 2000 Mar;74(2-3):265-72. doi: 10.1016/s0031-6865(99)00050-3. Pharm Acta Helv. 2000. PMID: 10812968 Review.
-
Humanin and the receptors for humanin.Mol Neurobiol. 2010 Feb;41(1):22-8. doi: 10.1007/s12035-009-8090-z. Epub 2009 Dec 9. Mol Neurobiol. 2010. PMID: 19997871 Review.
Cited by
-
Humanin: a harbinger of mitochondrial-derived peptides?Trends Endocrinol Metab. 2013 May;24(5):222-8. doi: 10.1016/j.tem.2013.01.005. Epub 2013 Feb 8. Trends Endocrinol Metab. 2013. PMID: 23402768 Free PMC article.
-
Efficacy of a Novel Mitochondrial-Derived Peptide in a Porcine Model of Myocardial Ischemia/Reperfusion Injury.JACC Basic Transl Sci. 2020 Jun 17;5(7):699-714. doi: 10.1016/j.jacbts.2020.04.015. eCollection 2020 Jul. JACC Basic Transl Sci. 2020. PMID: 32760857 Free PMC article.
-
Central effects of humanin on hepatic triglyceride secretion.Am J Physiol Endocrinol Metab. 2015 Aug 1;309(3):E283-92. doi: 10.1152/ajpendo.00043.2015. Epub 2015 Jun 9. Am J Physiol Endocrinol Metab. 2015. PMID: 26058861 Free PMC article.
-
Murine maternal dietary restriction affects neural Humanin expression and cellular profile.J Neurosci Res. 2020 May;98(5):902-920. doi: 10.1002/jnr.24568. Epub 2019 Dec 15. J Neurosci Res. 2020. PMID: 31840315 Free PMC article.
-
Humanin protects cortical neurons from ischemia and reperfusion injury by the increased activity of superoxide dismutase.Neurochem Res. 2012 Jan;37(1):153-60. doi: 10.1007/s11064-011-0593-0. Epub 2011 Sep 21. Neurochem Res. 2012. PMID: 21935731
References
-
- Benaki D., Zikos C., Evangelou A., Livaniou E., Vlassi M., Mikros E., Pelecanou M. Solution structure of humanin, a peptide against Alzheimer's disease-related neurotoxicity. Biochem. Biophys. Res. Commun. 2005;329:152–160. - PubMed
-
- Bozyczko-Coyne D., McKenna B. W., Connors T. J., Neff N. T. A rapid fluorometric assay to measure neuronal survival in vitro. J. Neurosci. Methods. 1993;50:205–216. - PubMed
-
- Boulanger M. J., Garcia K. C. Shared cytokine signaling receptors: structural insights from the gp130 system. Adv. Protein Chem. 2004;68:107–146. - PubMed
-
- Boulay J-L, O'Shea J. J., Paul W. E. Molecular phylogeny within type I cytokines and their cognate receptors. Immunity. 2003;19:159–163. - PubMed
-
- Caricasole A., Bruno V., Cappuccio I., Melchiorri D., Copani A., Nicoletti F. A novel rat gene encoding a Humanin-like peptide endowed with broad neuroprotective activity. FASEB J. 2002;16:1331–1333. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

