Variations in the composition of gelling agents affect morphophysiological and molecular responses to deficiencies of phosphate and other nutrients
- PMID: 19386810
- PMCID: PMC2689959
- DOI: 10.1104/pp.109.136184
Variations in the composition of gelling agents affect morphophysiological and molecular responses to deficiencies of phosphate and other nutrients
Abstract
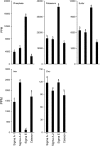
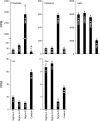
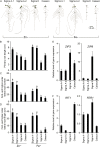
Low inorganic phosphate (Pi) availability triggers an array of spatiotemporal adaptive responses in Arabidopsis (Arabidopsis thaliana). There are several reports on the effects of Pi deprivation on the root system that have been attributed to different growth conditions and/or inherent genetic variability. Here we show that the gelling agents, largely treated as inert components, significantly affect morphophysiological and molecular responses of the seedlings to deficiencies of Pi and other nutrients. Inductively coupled plasma-mass spectroscopy analysis revealed variable levels of elemental contaminants not only in different types of agar but also in different batches of the same agar. Fluctuating levels of phosphorus (P) in different agar types affected the growth of the seedlings under Pi-deprivation condition. Since P interacts with other elements such as iron, potassium, and sulfur, contaminating effects of these elements in different agars were also evident in the Pi-deficiency-induced morphological and molecular responses. P by itself acted as a contaminant when studying the responses of Arabidopsis to micronutrient (iron and zinc) deficiencies. Together, these results highlighted the likelihood of erroneous interpretations that could be easily drawn from nutrition studies when different agars have been used. As an alternative, we demonstrate the efficacy of a sterile and contamination-free hydroponic system for dissecting morphophysiological and molecular responses of Arabidopsis to different nutrient deficiencies.
Figures



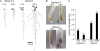

Similar articles
-
Plasticity of the Arabidopsis root system under nutrient deficiencies.Plant Physiol. 2013 Sep;163(1):161-79. doi: 10.1104/pp.113.218453. Epub 2013 Jul 12. Plant Physiol. 2013. PMID: 23852440 Free PMC article.
-
Differential effects of sucrose and auxin on localized phosphate deficiency-induced modulation of different traits of root system architecture in Arabidopsis.Plant Physiol. 2007 May;144(1):232-47. doi: 10.1104/pp.106.092130. Epub 2007 Mar 16. Plant Physiol. 2007. PMID: 17369438 Free PMC article.
-
Adaptation to Phosphate Scarcity: Tips from Arabidopsis Roots.Trends Plant Sci. 2018 Aug;23(8):721-730. doi: 10.1016/j.tplants.2018.04.006. Epub 2018 May 12. Trends Plant Sci. 2018. PMID: 29764728 Review.
-
Phosphate-Dependent Root System Architecture Responses to Salt Stress.Plant Physiol. 2016 Oct;172(2):690-706. doi: 10.1104/pp.16.00712. Epub 2016 May 20. Plant Physiol. 2016. PMID: 27208277 Free PMC article.
-
Root developmental adaptation to phosphate starvation: better safe than sorry.Trends Plant Sci. 2011 Aug;16(8):442-50. doi: 10.1016/j.tplants.2011.05.006. Trends Plant Sci. 2011. PMID: 21684794 Review.
Cited by
-
Buffered delivery of phosphate to Arabidopsis alters responses to low phosphate.J Exp Bot. 2018 Feb 23;69(5):1207-1219. doi: 10.1093/jxb/erx454. J Exp Bot. 2018. PMID: 29304231 Free PMC article.
-
Vigorous Growing of Donor Plantlets by Liquid Overlay in Subcultures Is the Key to Cryopreservation of Endangered Species Pogostemon yatabeanus.Plants (Basel). 2022 Nov 16;11(22):3127. doi: 10.3390/plants11223127. Plants (Basel). 2022. PMID: 36432856 Free PMC article.
-
Plasticity of the Arabidopsis root system under nutrient deficiencies.Plant Physiol. 2013 Sep;163(1):161-79. doi: 10.1104/pp.113.218453. Epub 2013 Jul 12. Plant Physiol. 2013. PMID: 23852440 Free PMC article.
-
Ethylene Response Factor070 regulates root development and phosphate starvation-mediated responses.Plant Physiol. 2014 Mar;164(3):1484-98. doi: 10.1104/pp.113.231183. Epub 2014 Jan 6. Plant Physiol. 2014. PMID: 24394776 Free PMC article.
-
Deciphering Phosphate Deficiency-Mediated Temporal Effects on Different Root Traits in Rice Grown in a Modified Hydroponic System.Front Plant Sci. 2016 May 4;7:550. doi: 10.3389/fpls.2016.00550. eCollection 2016. Front Plant Sci. 2016. PMID: 27200025 Free PMC article.
References
-
- Al-Ghazi Y, Muller B, Pinloche S, Tranbarger TJ, Nacry P, Rossignol M, Tardieu F, Doumas P (2003) Temporal responses of Arabidopsis root architecture to phosphate starvation: evidence for the involvement of auxin signaling. Plant Cell Environ 26 1053–1066
-
- Beruto M, Beruto D, Debergh P (1999) Influence of agar on in vitro cultures. I. Physicochemical properties of agar and agar gelled media. In Vitro Cell Dev Biol Plant 35 86–93
-
- Chen DL, Delatorre CA, Bakker A, Abel S (2000) Conditional identification of phosphate starvation-response mutants in Arabidopsis thaliana. Planta 211 13–22 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

