Complex intrinsic membrane properties and dopamine shape spiking activity in a motor axon
- PMID: 19386902
- PMCID: PMC2743087
- DOI: 10.1523/JNEUROSCI.0716-09.2009
Complex intrinsic membrane properties and dopamine shape spiking activity in a motor axon
Abstract
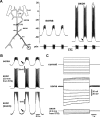
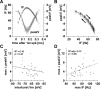
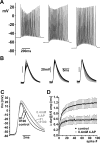
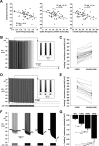
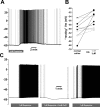
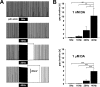
We studied the peripheral motor axons of the two pyloric dilator (PD) neurons of the stomatogastric ganglion in the lobster, Homarus americanus. Intracellular recordings from the motor nerve showed both fast and slow voltage- and activity-dependent dynamics. During rhythmic bursts, the PD axons displayed changes in spike amplitude and duration. Pharmacological experiments and the voltage dependence of these phenomena suggest that inactivation of sodium and A-type potassium channels are responsible. In addition, the "resting" membrane potential was dependent on ongoing spike or burst activity, with more hyperpolarized values when activity was strong. Nerve stimulations, pharmacological block and current clamp experiments suggest that this is due to a functional antagonism between a slow after-hyperpolarization (sAHP) and inward rectification through hyperpolarization-activated current (IH). Dopamine application resulted in modest depolarization and "ectopic" peripheral spike initiation in the absence of centrally generated activity. This effect was blocked by CsCl and ZD7288, consistent with a role of IH. High frequency nerve stimulation inhibited peripheral spike initiation for several seconds, presumably due to the sAHP. Both during normal bursting activity and antidromic nerve stimulation, the conduction delay over the length of the peripheral nerve changed in a complex manner. This suggests that axonal membrane dynamics can have a substantial effect on the temporal fidelity of spike patterns propagated from a spike initiation site to a synaptic target, and that neuromodulators can influence the extent to which spike patterns are modified.
Figures


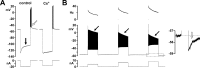


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
