Brain injury does not alter the intrinsic differentiation potential of adult neuroblasts
- PMID: 19386903
- PMCID: PMC6665479
- DOI: 10.1523/JNEUROSCI.0201-09.2009
Brain injury does not alter the intrinsic differentiation potential of adult neuroblasts
Abstract
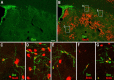
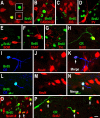
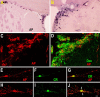
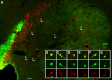
Neuroblasts produced by the neural stem cells of the adult subventricular zone (SVZ) migrate into damaged brain areas after stroke or other brain injuries, and previous data have suggested that they generate regionally appropriate new neurons. To classify the types of neurons produced subsequent to ischemic injury, we combined BrdU or virus labeling with multiple neuronal markers to characterize new cells at different times after the induction of stroke. We show that SVZ neuroblasts give rise almost exclusively to calretinin-expressing cells in the damaged striatum, resulting in the accumulation of these cells during long term recovery after stroke. The vast majority of SVZ neuroblasts as well as newly born young and mature neurons in the damaged striatum constitutively express the transcription factor Sp8, but do not express transcription factors characteristic of medium-sized spiny neurons, the primary striatal projection neurons lost after stroke. Our results suggest that adult neuroblasts do not alter their intrinsic differentiation potential after brain injury.
Figures


Similar articles
-
Neurogenesis in the striatum of the quinolinic acid lesion model of Huntington's disease.Neuroscience. 2004;127(2):319-32. doi: 10.1016/j.neuroscience.2004.04.061. Neuroscience. 2004. PMID: 15262322
-
Absence of striatal newborn neurons with mature phenotype following defined striatal and cortical excitotoxic brain injuries.Exp Neurol. 2009 Sep;219(1):363-7. doi: 10.1016/j.expneurol.2009.05.002. Epub 2009 May 8. Exp Neurol. 2009. PMID: 19427853
-
Rat forebrain neurogenesis and striatal neuron replacement after focal stroke.Ann Neurol. 2002 Dec;52(6):802-13. doi: 10.1002/ana.10393. Ann Neurol. 2002. PMID: 12447935
-
Forebrain neurogenesis after focal Ischemic and traumatic brain injury.Neurobiol Dis. 2010 Feb;37(2):267-74. doi: 10.1016/j.nbd.2009.11.002. Epub 2009 Nov 10. Neurobiol Dis. 2010. PMID: 19909815 Free PMC article. Review.
-
Injury-induced neurogenesis in the adult mammalian brain.Neuroscientist. 2003 Aug;9(4):261-72. doi: 10.1177/1073858403252680. Neuroscientist. 2003. PMID: 12934709 Review.
Cited by
-
Excitable Adult-Generated GABAergic Neurons Acquire Functional Innervation in the Cortex after Stroke.Stem Cell Reports. 2018 Dec 11;11(6):1327-1336. doi: 10.1016/j.stemcr.2018.10.011. Epub 2018 Nov 8. Stem Cell Reports. 2018. PMID: 30416050 Free PMC article.
-
New striatal neurons in a mouse model of progressive striatal degeneration are generated in both the subventricular zone and the striatal parenchyma.PLoS One. 2011;6(9):e25088. doi: 10.1371/journal.pone.0025088. Epub 2011 Sep 30. PLoS One. 2011. PMID: 21980380 Free PMC article.
-
Noncanonical Sites of Adult Neurogenesis in the Mammalian Brain.Cold Spring Harb Perspect Biol. 2015 Sep 18;7(10):a018846. doi: 10.1101/cshperspect.a018846. Cold Spring Harb Perspect Biol. 2015. PMID: 26384869 Free PMC article. Review.
-
Transcription Factor-Based Gene Therapy Enables Functional Repair of Rat Following Chronic Ischemic Stroke.CNS Neurosci Ther. 2025 May;31(5):e70448. doi: 10.1111/cns.70448. CNS Neurosci Ther. 2025. PMID: 40401537 Free PMC article.
-
New neurons in the adult striatum: from rodents to humans.Trends Neurosci. 2015 Sep;38(9):517-23. doi: 10.1016/j.tins.2015.07.005. Epub 2015 Aug 20. Trends Neurosci. 2015. PMID: 26298770 Free PMC article. Review.
References
-
- Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to sonic hedgehog. Nature. 2005;437:894–897. - PubMed
-
- Allen ZJ, 2nd, Waclaw RR, Colbert MC, Campbell K. Molecular identity of olfactory bulb interneurons: transcriptional codes of periglomerular neuron subtypes. J Mol Histol. 2007;38:517–525. - PubMed
-
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137:433–457. - PubMed
-
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
