Dopamine modulation of GABA tonic conductance in striatal output neurons
- PMID: 19386907
- PMCID: PMC2707274
- DOI: 10.1523/JNEUROSCI.4737-08.2009
Dopamine modulation of GABA tonic conductance in striatal output neurons
Abstract
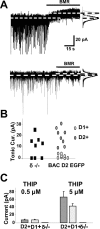
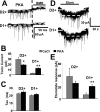
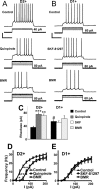
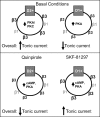
We previously reported greater GABAA receptor-mediated tonic currents in D2+ striatopallidal than D1+ striatonigral medium spiny neurons (MSNs) are mediated by alpha5-subunit-containing receptors. Here, we used whole-cell recordings in slices from bacterial artificial chromosome transgenic mice to investigate the link between subunit composition, phosphorylation, and dopamine receptor activation. Whole-cell recordings in slices from delta-subunit knock-out mice demonstrate that while MSNs in wild-type mice do express delta-subunit-containing receptors, this receptor subtype is not responsible for tonic conductance observed in the acute slice preparation. We assessed the contribution of the beta1- and beta3-subunits expressed in MSNs by their sensitivity to etomidate, an agonist selective for beta2- or beta3-subunit-containing GABAA receptors. Although etomidate produced substantial tonic current in D2+ neurons, there was no effect in D1+ neurons. However, with internal PKA application or dopamine modulation, D1+ neurons expressed tonic conductance and responded to etomidate application. Our results suggest that distinct phosphorylation of beta3-subunits may cause larger tonic current in D2+ striatopallidal MSNs, and proper intracellular conditions can reveal tonic current in D1+ cells.
Figures




Similar articles
-
Distinct roles of synaptic and extrasynaptic GABAAreceptors in striatal inhibition dynamics.Front Neural Circuits. 2013 Nov 26;7:186. doi: 10.3389/fncir.2013.00186. eCollection 2013. Front Neural Circuits. 2013. PMID: 24324406 Free PMC article.
-
Differential tonic GABA conductances in striatal medium spiny neurons.J Neurosci. 2008 Jan 30;28(5):1185-97. doi: 10.1523/JNEUROSCI.3908-07.2008. J Neurosci. 2008. PMID: 18234896 Free PMC article.
-
Developmental regulation and neuroprotective effects of striatal tonic GABAA currents.Neuroscience. 2010 May 19;167(3):644-55. doi: 10.1016/j.neuroscience.2010.02.048. Epub 2010 Mar 3. Neuroscience. 2010. PMID: 20206233 Free PMC article.
-
D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons.Trends Neurosci. 2007 May;30(5):228-35. doi: 10.1016/j.tins.2007.03.008. Epub 2007 Apr 3. Trends Neurosci. 2007. PMID: 17408758 Review.
-
Regulation of corticostriatal synaptic plasticity by G protein-coupled receptors.CNS Neurol Disord Drug Targets. 2010 Nov;9(5):601-15. doi: 10.2174/187152710793361531. CNS Neurol Disord Drug Targets. 2010. PMID: 20632967 Review.
Cited by
-
GABAA receptor-mediated tonic depolarization in developing neural circuits.Mol Neurobiol. 2014 Apr;49(2):702-23. doi: 10.1007/s12035-013-8548-x. Epub 2013 Sep 11. Mol Neurobiol. 2014. PMID: 24022163 Review.
-
Detection of phasic dopamine by D1 and D2 striatal medium spiny neurons.J Physiol. 2017 Dec 15;595(24):7451-7475. doi: 10.1113/JP274475. Epub 2017 Sep 2. J Physiol. 2017. PMID: 28782235 Free PMC article.
-
Inflammation alters AMPA-stimulated calcium responses in dorsal striatal D2 but not D1 spiny projection neurons.Eur J Neurosci. 2017 Nov;46(9):2519-2533. doi: 10.1111/ejn.13711. Epub 2017 Oct 10. Eur J Neurosci. 2017. PMID: 28921719 Free PMC article.
-
What is the Degree of Segregation between Striatonigral and Striatopallidal Projections?Front Neuroanat. 2010 Oct 7;4:136. doi: 10.3389/fnana.2010.00136. eCollection 2010. Front Neuroanat. 2010. PMID: 20953289 Free PMC article.
-
Cortical and thalamic innervation of direct and indirect pathway medium-sized spiny neurons in mouse striatum.J Neurosci. 2010 Nov 3;30(44):14610-8. doi: 10.1523/JNEUROSCI.1623-10.2010. J Neurosci. 2010. PMID: 21048118 Free PMC article.
References
-
- Belousov AB, van den Pol AN. Dopamine inhibition: enhancement of GABA activity and potassium channel activation in hypothalamic and arcuate nucleus neurons. J Neurophysiol. 1997;78:674–688. - PubMed
-
- Brandon NJ, Delmas P, Kittler JT, McDonald BJ, Sieghart W, Brown DA, Smart TG, Moss SJ. GABAA receptor phosphorylation and functional modulation in cortical neurons by a protein kinase C-dependent pathway. J Biol Chem. 2000;275:38856–38862. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
