NAD+ activates KNa channels in dorsal root ganglion neurons
- PMID: 19386908
- PMCID: PMC6665483
- DOI: 10.1523/JNEUROSCI.0859-09.2009
NAD+ activates KNa channels in dorsal root ganglion neurons
Abstract
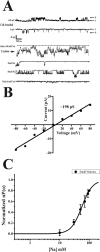
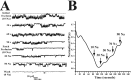
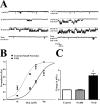
Although sodium-activated potassium channels (KNa) have been suggested to shape various firing patterns in neurons, including action potential repolarization, their requirement for high concentrations of Na+ to gate conflicts with this view. We characterized KNa channels in adult rat dorsal root ganglion (DRG) neurons. Using immunohistochemistry, we found ubiquitous expression of the Slack KNa channel subunit in small-, medium-, and large-diameter DRG neurons. Basal KNa channel activity could be recorded from cell-attached patches of acutely dissociated neurons bathed in physiological saline, and yet in excised inside-out membrane patches, the Na+ EC50 for KNa channels was typically high, approximately 50 mM. In some cases, however, KNa channel activity remained considerable after initial patch excision but decreased rapidly over time. Channel activity was restored in patches with high Na+. The channel rundown after initial excision suggested that modulation of channels might be occurring through a diffusible cytoplasmic factor. Sequence analysis indicated that the Slack channel contains a putative nicotinamide adenine dinucleotide (NAD+)-binding site; accordingly, we examined the modulation of native KNa and Slack channels by NAD+. In inside-out-excised neuronal patch recordings, we found a decrease in the Na+ EC50 for KNa channels from approximately 50 to approximately 20 mM when NAD+ was included in the perfusate. NAD+ also potentiated recombinant Slack channel activity. NAD+ modulation may allow KNa channels to operate under physiologically relevant levels of intracellular Na+ and hence provides an explanation as to how KNa channel can control normal neuronal excitability.
Figures



References
-
- Bellamacina CR. The nicotinamide dinucleotide binding motif: a comparison of nucleotide binding proteins. FASEB J. 1996;10:1257–1269. - PubMed
-
- Bhattacharjee A, Kaczmarek LK. For K+ channels, Na+ is the new Ca2+ Trends Neurosci. 2005;28:422–428. - PubMed
-
- Bhattacharjee A, Gan L, Kaczmarek LK. Localization of the Slack potassium channel in the rat central nervous system. J Comp Neurol. 2002;454:241–254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
