Efficiency and safety of AAV-mediated gene delivery of the human ND4 complex I subunit in the mouse visual system
- PMID: 19387075
- PMCID: PMC3085487
- DOI: 10.1167/iovs.08-3214
Efficiency and safety of AAV-mediated gene delivery of the human ND4 complex I subunit in the mouse visual system
Abstract
Purpose: To evaluate the efficiency and safety of AAV-mediated gene delivery of a normal human ND4 complex I subunit in the mouse visual system.
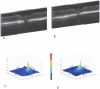
Methods: A nuclear encoded human ND4 subunit fused to the ATPc mitochondrial targeting sequence and FLAG epitope were packaged in AAV2 capsids that were injected into the right eyes of mice. AAV-GFP was injected into the left eyes. One month later, pattern electroretinography (PERG), rate of ATP synthesis, gene expression, and incorporation of the human ND4 subunit into the murine complex I were evaluated. Quantitative analysis of ND4FLAG-injected eyes was assessed compared with green fluorescent protein (GFP)-injected eyes.
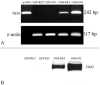
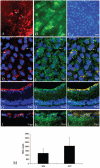
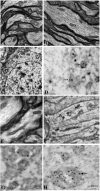
Results: Rates of ATP synthesis and PERG amplitudes were similar in ND4FLAG- and GFP-inoculated eyes. PERG latency was shorter in eyes that received ND4FLAG. Immunoprecipitated murine complex I gave the expected 52-kDa band of processed human ND4FLAG. Confocal microscopy revealed perinuclear expression of FLAG colocalized with mitochondria-specific fluorescent dye. Transmission electron microscopy revealed FLAG immunogold within mitochondria. Compared with Thy1.2-positive retinal ganglion cells (RGCs), quantification was 38% for FLAG-positive RGCs and 65% for GFP-positive RGCs. Thy1.2 positive-RGC counts in AAV-ND4FLAG were similar to counts in control eyes injected with AAV-GFP.
Conclusions: Human ND4 was properly processed and imported into the mitochondria of RGCs and axons of mouse optic nerve after intravitreal injection. Although it had approximately two-thirds the efficiency of GFP, the expression of normal human ND4 in murine mitochondria did not induce the loss of RGCs, ATP synthesis, or PERG amplitude, suggesting that allotopic ND4 may be safe for the treatment of patients with Leber hereditary optic neuropathy.
Figures



References
-
- Wallace DC, Singh G, Lott MT, et al. Mitochondrial DNA mutation associated with Leber hereditary optic neuropathy. Science. 1988;242:1427–1430. - PubMed
-
- Carelli V, Ghelli A, Bucchi L, et al. Biochemical features of mtDNA 14484 (ND6/M64V) point mutation associated with Leber’s hereditary optic neuropathy. Ann Neurol. 1999;45:320–328. - PubMed
-
- Glick B, Schatz G. Import of proteins into mitochondria. Annu Rev Genet. 1991;25:21–44. - PubMed
-
- Neupert W. Protein import into mitochondria. Annu Rev Biochem. 1997;66:863–917. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

