Prevention of pancreatic cancer by the beta-blocker propranolol
- PMID: 19387337
- PMCID: PMC3366433
- DOI: 10.1097/CAD.0b013e32832bd1e3
Prevention of pancreatic cancer by the beta-blocker propranolol
Abstract
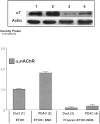
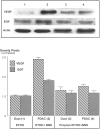
Pancreatic ductal adenocarcinoma (PDAC) is among the leading causes of cancer deaths and is unresponsive to existing therapy. Smoking and alcohol-induced pancreatitis are among the risk factors for PDAC. We have previously reported that beta-adrenergic receptors (beta-ARs) stimulate the proliferation and migration of human PDAC cells in vitro by cAMP-dependent signaling and that the nicotine-derived nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) activates this pathway directly in vitro while additionally stimulating the release of noradrenaline/adrenaline by binding to alpha7 nicotinic acetylcholine receptors (alpha7 nAChR) in hamsters. In this study, we have tested the hypothesis that the beta-AR antagonist propranolol prevents the development of PDAC induced in hamsters with ethanol-induced pancreatitis by NNK. We found that propranolol had strong cancer preventive effects in this animal model. Western blots of pancreatic duct cells and PDAC cells harvested by laser capture microscopy showed significant upregulation of the alpha7 nAChR associated with significant inductions of p-CREB, p-ERK1/2, and increases in epidermal growth factor and vascular endothelial growth factor in PDAC cells of hamsters not treated with propranolol. These effects were reversed by treatment with propranolol. Our data suggest that propranolol may prevent the development of PDAC by blocking cAMP-dependent intracellular signaling, cAMP-dependent release of epidermal growth factor, and PKA-dependent release of vascular endothelial growth factor while additionally downregulating the alpha7 nAChR by inhibiting cAMP-mediated subunit assembly. We conclude that increased cAMP signaling is an important factor that drives the development and progression of PDAC and that the inhibition of cAMP formation is a promising new target for the prevention and adjuvant therapy of PDAC.
Figures


References
-
- Mancuso A, Calabro F, Sternberg CN. Current therapies and advances in the treatment of pancreatic cancer. Crit Rev Oncol Hematol. 2006;58:231–41. - PubMed
-
- Lowenfels AB, Maisonneuve P. Risk factors for pancreatic cancer. J Cell Biochem. 2005;95:649–56. - PubMed
-
- Weddle DL, Tithoff P, Williams M, Schuller HM. Beta-adrenergic growth regulation of human cancer cell lines derived from pancreatic ductal carcinomas. Carcinogenesis. 2001;22:473–9. - PubMed
-
- Askari MD, Tsao MS, Schuller HM. The tobacco-specific carcinogen, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone stimulates proliferation of immortalized human pancreatic duct epithelia through beta-adrenergic transactivation of EGF receptors. J Cancer Res Clin Oncol. 2005;131:639–48. - PubMed
-
- Jin Z, Gao F, Flagg T, Deng X. Tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone promotes functional cooperation of Bcl2 and c-Myc through phosphorylation in regulating cell survival and proliferation. J Biol Chem. 2004;279:40209–19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

