NAADP mobilizes calcium from acidic organelles through two-pore channels
- PMID: 19387438
- PMCID: PMC2761823
- DOI: 10.1038/nature08030
NAADP mobilizes calcium from acidic organelles through two-pore channels
Abstract
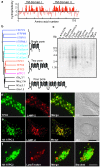
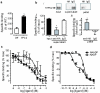
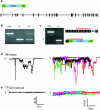
Ca(2+) mobilization from intracellular stores represents an important cell signalling process that is regulated, in mammalian cells, by inositol-1,4,5-trisphosphate (InsP(3)), cyclic ADP ribose and nicotinic acid adenine dinucleotide phosphate (NAADP). InsP(3) and cyclic ADP ribose cause the release of Ca(2+) from sarcoplasmic/endoplasmic reticulum stores by the activation of InsP(3) and ryanodine receptors (InsP(3)Rs and RyRs). In contrast, the nature of the intracellular stores targeted by NAADP and the molecular identity of the NAADP receptors remain controversial, although evidence indicates that NAADP mobilizes Ca(2+) from lysosome-related acidic compartments. Here we show that two-pore channels (TPCs) comprise a family of NAADP receptors, with human TPC1 (also known as TPCN1) and chicken TPC3 (TPCN3) being expressed on endosomal membranes, and human TPC2 (TPCN2) on lysosomal membranes when expressed in HEK293 cells. Membranes enriched with TPC2 show high affinity NAADP binding, and TPC2 underpins NAADP-induced Ca(2+) release from lysosome-related stores that is subsequently amplified by Ca(2+)-induced Ca(2+) release by InsP(3)Rs. Responses to NAADP were abolished by disrupting the lysosomal proton gradient and by ablating TPC2 expression, but were only attenuated by depleting endoplasmic reticulum Ca(2+) stores or by blocking InsP(3)Rs. Thus, TPCs form NAADP receptors that release Ca(2+) from acidic organelles, which can trigger further Ca(2+) signals via sarcoplasmic/endoplasmic reticulum. TPCs therefore provide new insights into the regulation and organization of Ca(2+) signals in animal cells, and will advance our understanding of the physiological role of NAADP.
Figures

References
-
- Berridge MJ, Bootman MD, Roderick HL. Calcium: Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003;4:517–529. - PubMed
-
- Galione A, Churchill GC. Interactions between calcium release pathways: multiple messengers and multiple stores. Cell Calcium. 2002;32:343–354. - PubMed
-
- Churchill GC, et al. NAADP mobilizes Ca2+ from reserve granules, a lysosome-related organelle, in sea urchin eggs. Cell. 2002;111:703–708. - PubMed
-
- Yamasaki M, et al. Organelle selection determines agonist-specific Ca2+ signals in pancreatic acinar and beta cells. J. Biol. Chem. 2004;279:7234–7240. - PubMed
-
- Lee HC, Aarhus R. A derivative of NADP mobilizes calcium stores insensitive to inositol trisphosphate and cyclic ADP-ribose. J. Biol. Chem. 1995;270:2152–2157. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

