A dietary non-human sialic acid may facilitate hemolytic-uremic syndrome
- PMID: 19387473
- PMCID: PMC2752721
- DOI: 10.1038/ki.2009.131
A dietary non-human sialic acid may facilitate hemolytic-uremic syndrome
Abstract
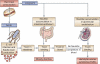
Hemolytic-uremic syndrome (HUS) is a systemic disease characterized by microvascular endothelial damage, mainly in the gastrointestinal tract and the kidneys. A major cause of HUS is Shiga toxigenic Escherichia coli (STEC) infection. In addition to Shiga toxin, additional STEC virulence factors may contribute to HUS. One is the newly discovered subtilase cytotoxin (SubAB), which is highly toxic to eukaryotic cells, and when injected intraperitoneally into mice causes pathology resembling that associated with human HUS. Recent data show that SubAB exhibits a strong preference for glycans terminating in alpha2-3-linked N-glycolylneuraminic acid (Neu5Gc), a sialic acid that humans are unable to synthesize, because we genetically lack the necessary enzyme. However, Neu5Gc can still be found on human cells due to metabolic incorporation from the diet. Dietary incorporation happens to be highest in human endothelium and to a lesser extent in the intestinal epithelium, the two affected cell types in STEC-induced HUS. Mammalian-derived foods such as red meat and dairy products appear to be the primary source of dietary Neu5Gc. Ironically, these are also common sources of STEC contamination. Taken together, these findings suggest a 'two-hit' process in the pathogenesis of human SubAB-induced disease. First, humans eat Neu5Gc-rich food, leading to incorporation of Neu5Gc on the surfaces of endothelial and intestinal cells. Second, when exposed to a SubAB-producing STEC strain, the toxin produced would be able to bind to the intestinal epithelial cells, perhaps causing acute gastrointestinal symptoms, and eventually damaging endothelial cells in other organs like the kidney, thereby causing HUS.
Figures

References
-
- Karmali MA, Petric M, Lim C, et al. The association between idiopathic hemolytic uremic syndrome and infection by verotoxin-producing Escherichia coli. J Infect Dis. 1985;151:775–782. - PubMed
-
- Tarr PI, Gordon CA, Chandler WL. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet. 2005;365:1073–1086. - PubMed
-
- Fiorino EK, Raffaelli RM. Hemolytic-uremic syndrome. Pediatr Rev. 2006;27:398–399. discussion 399. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

