Key role of the N-terminus of chicken annexin A5 in vesicle aggregation
- PMID: 19388055
- PMCID: PMC2771311
- DOI: 10.1002/pro.119
Key role of the N-terminus of chicken annexin A5 in vesicle aggregation
Abstract
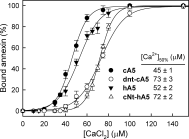
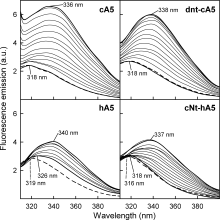
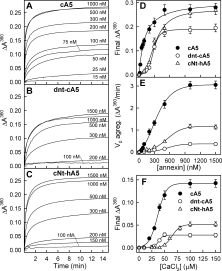
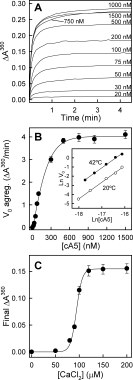
Annexins are calcium-dependent phospholipid-binding proteins involved in calcium signaling and intracellular membrane trafficking among other functions. Vesicle aggregation is a crucial event to make possible the membrane remodeling but this process is energetically unfavorable, and phospholipid membranes do not aggregate and fuse spontaneously. This issue can be circumvented by the presence of different agents such as divalent cations and/or proteins, among them some annexins. Although human annexin A5 lacks the ability to aggregate vesicles, here we demonstrate that its highly similar chicken ortholog induces aggregation of vesicles containing acidic phospholipids even at low protein and/or calcium concentration by establishment of protein dimers. Our experiments show that the ability to aggregate vesicles mainly resides in the N-terminus as truncation of the N-terminus of chicken annexin A5 significantly decreases this process and replacement of the N-terminus of human annexin A5 by that of chicken switches on aggregation; in both cases, there are no changes in the overall protein structure and only minor changes in phospholipid binding. Electrostatic repulsions between negatively charged residues in the concave face of the molecule, mainly in the N-terminus, seem to be responsible for the impairment of dimer formation in human annexin A5. Taking into account that chicken annexin A5 presents a high sequence and structural similarity with mammalian annexins absent in birds, as annexins A3 and A4, some of the physiological functions exerted by these proteins may be carried out by chicken annexin A5, even those that could require calcium-dependent membrane aggregation.
Figures






Similar articles
-
Annexin A5 stabilizes matrix vesicle-biomimetic lipid membranes: unravelling a new role of annexins in calcification.Eur Biophys J. 2023 Nov;52(8):721-733. doi: 10.1007/s00249-023-01687-4. Epub 2023 Nov 8. Eur Biophys J. 2023. PMID: 37938350 Free PMC article.
-
Aggregation of phospholipid vesicles by a chimeric protein with the N-terminus of annexin I and the core of annexin V.Biochemistry. 1993 May 4;32(17):4634-40. doi: 10.1021/bi00068a022. Biochemistry. 1993. PMID: 8485141
-
The conserved core domains of annexins A1, A2, A5, and B12 can be divided into two groups with different Ca2+-dependent membrane-binding properties.Biochemistry. 2005 Mar 1;44(8):2833-44. doi: 10.1021/bi047642+. Biochemistry. 2005. PMID: 15723527
-
Annexin V and phospholipid metabolism.Clin Chem Lab Med. 1999 Mar;37(3):287-91. doi: 10.1515/CCLM.1999.050. Clin Chem Lab Med. 1999. PMID: 10353474 Review.
-
Annexin V-crystal structure and its implications on function.Behring Inst Mitt. 1992 Apr;(91):107-25. Behring Inst Mitt. 1992. PMID: 1388018 Review.
Cited by
-
Annexin-phospholipid interactions. Functional implications.Int J Mol Sci. 2013 Jan 28;14(2):2652-83. doi: 10.3390/ijms14022652. Int J Mol Sci. 2013. PMID: 23358253 Free PMC article.
-
Molecular dynamics study of naturally existing cavity couplings in proteins.PLoS One. 2015 Mar 27;10(3):e0119978. doi: 10.1371/journal.pone.0119978. eCollection 2015. PLoS One. 2015. PMID: 25816327 Free PMC article.
References
-
- Gerke V, Moss SE. Annexins: from structure to function. Physiol Rev. 2002;82:331–371. - PubMed
-
- Gerke V, Creutz CE, Moss SE. Annexins: linking Ca2+ signalling to membrane dynamics. Nat Rev Mol Cell Biol. 2005;6:449–461. - PubMed
-
- Turnay J, Lecona E, Guzmán-Aránguez A, Pérez-Ramos P, Fernández-Lizarbe S, Olmo N, Lizarbe MA. Annexins: structural characteristics of the N-terminus and influence and influence on the overall structure of the protein. Recent Res Dev Biochem. 2003b;4:79–95.
-
- Turnay J, Guzmán-Aránguez A, Lecona E, Pérez-Ramos P, Fernández-Lizarbe S, Olmo N, Lizarbe MA. Influence of the N-terminal domain of annexins in their functional properties. Recent Res Dev Biochem. 2003a;4:53–78.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

