MRI and histological analysis of beta-amyloid plaques in both human Alzheimer's disease and APP/PS1 transgenic mice
- PMID: 19388095
- PMCID: PMC2723054
- DOI: 10.1002/jmri.21731
MRI and histological analysis of beta-amyloid plaques in both human Alzheimer's disease and APP/PS1 transgenic mice
Abstract
Purpose: To investigate the relationship between MR image contrast associated with beta-amyloid (Abeta) plaques and their histology and compare the histopathological basis of image contrast and the relaxation mechanism associated with Abeta plaques in human Alzheimer's disease (AD) and transgenic APP/PS1 mouse tissues.
Materials and methods: With the aid of the previously developed histological coil, T(2) (*)-weighted images and R(2) (*) parametric maps were directly compared with histology stains acquired from the same set of Alzheimer's and APP/PS1 tissue slices.
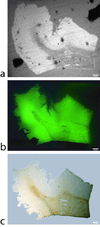
Results: The electron microscopy and histology images revealed significant differences in plaque morphology and associated iron concentration between AD and transgenic APP/PS1 mice tissue samples. For AD tissues, T(2) (*) contrast of Abeta-plaques was directly associated with the gradation of iron concentration. Plaques with significantly less iron load in the APP/PS1 animal tissues are equally conspicuous as the human plaques in the MR images.
Conclusion: These data suggest a duality in the relaxation mechanism where both high focal iron concentration and highly compact fibrillar beta-amyloid masses cause rapid proton transverse magnetization decay. For human tissues, the former mechanism is likely the dominant source of R(2) (*) relaxation; for APP/PS1 animals, the latter is likely the major cause of increased transverse proton relaxation rate in Abeta plaques. The data presented are essential for understanding the histopathological underpinning of MRI measurement associated with Abeta plaques in humans and animals.
Figures





References
-
- Jack CR, Jr, Marjanska M, Wengenack TM, Reyes DA, Curran GL, Lin J, Preboske GM, Poduslo JF, Garwood M. Magnetic resonance imaging of Alzheimer's pathology in the brains of living transgenic mice: a new tool in Alzheimer's disease research. Neuroscientist. 2007;13(1):38–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

