Hydroxyl radical oxidation of guanosine 5'-triphosphate (GTP): requirement for a GTP-Cu(II) complex
- PMID: 19389276
- PMCID: PMC2759603
- DOI: 10.1179/135100009X392520
Hydroxyl radical oxidation of guanosine 5'-triphosphate (GTP): requirement for a GTP-Cu(II) complex
Abstract
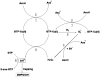
Levels of oxidized guanosine base in DNA have become a hallmark biomarker in assessing oxidative stress implicated in a variety of disease and toxin-induced states. However, there is evidence that the guanosine in the nucleotide triphosphate pool (GTP) is more susceptible to oxidation than guanosine residues incorporated into nucleic acids and this causes a substantial amount of the oxidized product, 8-oxoguanosine 5'-triphosphate (oxo(8)GTP), to accumulate in cell-free and in cell-culture preparations. Electron paramagnetic resonance (EPR) spectroscopy and direct EPR analysis of free radical production by copper sulfate and L-ascorbic acid demonstrates that the hydroxyl radical (HO(*)) is produced via oxidation of Cu(+) to Cu(2+) while in a complex with GTP. This HO(*) production is dependent on the availability of oxygen and the presence of GTP in the reaction milieu. Verification of free radical-mediated production of oxo(8)GTP is presented using HPLC with electrochemical detection and matrix-assisted laser desorption/ionization linear time-of-flight mass spectrometry (MALDI-LTOF-MS). The sum of these results is presented in a novel mechanism of GTP oxidation by Cu(2+) and L-ascorbic acid. A better understanding of the chemistry involved in this oxidative modification of GTP facilitates a more comprehensive understanding of its potential physiological consequences.
Figures

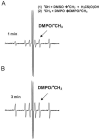



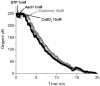
 ), in air-saturated PBS, pH 7.4 solution in a total cell volume of 1.5 mL at (22.0 ± 0.2) °C.
), in air-saturated PBS, pH 7.4 solution in a total cell volume of 1.5 mL at (22.0 ± 0.2) °C.
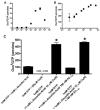
References
-
- Halliwell B, Gutteridge JM. Oxygen free radicals and iron in relation to biology and medicine: some problems and concepts. Arch Biochem Biophys. 1986;246(2):501–14. - PubMed
-
- Einolf HJ, Schnetz-Boutaud N, Guengerich FP. Steady-state and pre-steady-state kinetic analysis of 8-oxo-7,8-dihydroguanosine triphosphate incorporation and extension by replicative and repair DNA polymerases. Biochemistry. 1998;37(38):13300–12. - PubMed
-
- Hayakawa H, Taketomi A, Sakumi K, Kuwano M, Sekiguchi M. Generation and elimination of 8-oxo-7,8-dihydro-2′-deoxyguanosine 5′-triphosphate, a mutagenic substrate for DNA synthesis, in human cells. Biochemistry. 1995;34(1):89–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
