Residual microRNA expression dictates the extent of inner ear development in conditional Dicer knockout mice
- PMID: 19389351
- PMCID: PMC2793102
- DOI: 10.1016/j.ydbio.2009.01.037
Residual microRNA expression dictates the extent of inner ear development in conditional Dicer knockout mice
Abstract
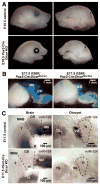
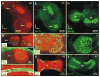
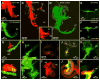
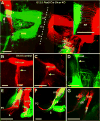
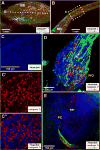
Inner ear development requires coordinated transformation of a uniform sheet of cells into a labyrinth with multiple cell types. While numerous regulatory proteins have been shown to play critical roles in this process, the regulatory functions of microRNAs (miRNAs) have not been explored. To demonstrate the importance of miRNAs in inner ear development, we generated conditional Dicer knockout mice by the expression of Cre recombinase in the otic placode at E8.5. Otocyst-derived ganglia exhibit rapid neuron-specific miR-124 depletion by E11.5, degeneration by E12.5, and profound defects in subsequent sensory epithelial innervations by E17.5. However, the small and malformed inner ear at E17.5 exhibits residual and graded hair cell-specific miR-183 expression in the three remaining sensory epithelia (posterior crista, utricle, and cochlea) that closely corresponds to the degree of hair cell and sensory epithelium differentiation, and Fgf10 expression required for morphohistogenesis. The highest miR-183 expression is observed in near-normal hair cells of the posterior crista, whereas the reduced utricular macula demonstrates weak miR-183 expression and develops presumptive hair cells with numerous disorganized microvilli instead of ordered stereocilia. The correlation of differential and delayed depletion of mature miRNAs with the derailment of inner ear development demonstrates that miRNAs are crucial for inner ear neurosensory development and neurosensory-dependent morphogenesis.
Figures




References
-
- Ahmed ZM, Goodyear R, Riazuddin S, Lagziel A, Legan PK, Behra M, Burgess SM, Lilley KS, Wilcox ER, Riazuddin S, Griffith AJ, Frolenkov GI, Belyantseva IA, Richardson GP, Friedman TB. The tip-link antigen, a protein associated with the transduction complex of sensory hair cells, is protocadherin-15. J Neurosci. 2006;26:7022–7034. - PMC - PubMed
-
- Amaral PP, Dinger ME, Mercer TR, Mattick JS. The eukaryotic genome is an RNA machine. Science. 2008;319:1787–1789. - PubMed
-
- Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, Mills AA, Elledge SJ, Anderson KV, Hannon GJ. Dicer is essential for mouse development. Nat Genet. 2003;35:215–217. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

