Bmi-1 over-expression in neural stem/progenitor cells increases proliferation and neurogenesis in culture but has little effect on these functions in vivo
- PMID: 19389366
- PMCID: PMC2996717
- DOI: 10.1016/j.ydbio.2009.01.020
Bmi-1 over-expression in neural stem/progenitor cells increases proliferation and neurogenesis in culture but has little effect on these functions in vivo
Abstract
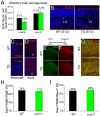
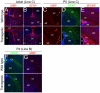
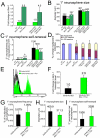
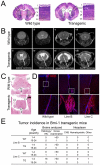
The polycomb gene Bmi-1 is required for the self-renewal of stem cells from diverse tissues, including the central nervous system (CNS). Bmi-1 expression is elevated in most human gliomas, irrespective of grade, raising the question of whether Bmi-1 over-expression is sufficient to promote self-renewal or tumorigenesis by CNS stem/progenitor cells. To test this we generated Nestin-Bmi-1-GFP transgenic mice. Analysis of two independent lines with expression in the fetal and adult CNS demonstrated that transgenic neural stem cells formed larger colonies, more self-renewing divisions, and more neurons in culture. However, in vivo, Bmi-1 over-expression had little effect on CNS stem cell frequency, subventricular zone proliferation, olfactory bulb neurogenesis, or neurogenesis/gliogenesis during development. Bmi-1 transgenic mice were born with enlarged lateral ventricles and a minority developed idiopathic hydrocephalus as adults, but none of the transgenic mice formed detectable CNS tumors, even when aged. The more pronounced effects of Bmi-1 over-expression in culture were largely attributable to the attenuated induction of p16(Ink4a) and p19(Arf) in culture, proteins that are generally not expressed by neural stem/progenitor cells in young mice in vivo. Bmi-1 over-expression therefore has more pronounced effects in culture and does not appear to be sufficient to induce tumorigenesis in vivo.
Figures
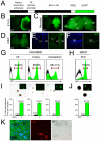
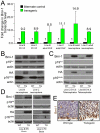
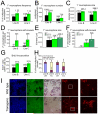
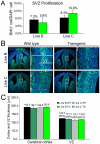
References
-
- Almqvist PM, Mah R, Lendahl U, Jacobsson B, Hendson G. Immunohistochemical detection of nestin in pediatric brain tumors. J Histochem Cytochem. 2002;50:147–58. - PubMed
-
- Bea S, Tort F, Pinyol M, Puig X, Hernandez L, Hernandez S, Fernandez PL, van Lohuizen M, Colomer D, Campo E. BMI-1 gene amplification and overexpression in hematological malignancies occur mainly in mantle cell lymphomas. Cancer Res. 2001;61:2409–12. - PubMed
-
- Ben-Saadon R, Zaaroor D, Ziv T, Ciechanover A. The polycomb protein Ring1B generates self atypical mixed ubiquitin chains required for its in vitro histone H2A ligase activity. Mol Cell. 2006;24:701–11. - PubMed
-
- Bruggeman SW, Hulsman D, Tanger E, Buckle T, Blom M, Zevenhoven J, van Tellingen O, van Lohuizen M. Bmi1 controls tumor development in an Ink4a/Arf-independent manner in a mouse model for glioma. Cancer Cell. 2007;12:328–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

