Single-pass, closed-system rapid expansion of lymphocyte cultures for adoptive cell therapy
- PMID: 19389403
- PMCID: PMC2700005
- DOI: 10.1016/j.jim.2009.04.009
Single-pass, closed-system rapid expansion of lymphocyte cultures for adoptive cell therapy
Abstract
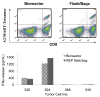
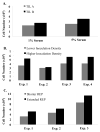
Adoptive cell therapy (ACT) for metastatic melanoma involves the ex vivo expansion and reinfusion of tumor infiltrating lymphocytes (TIL) obtained from resected specimens. With an overall objective response rate of 56%, this T-cell immunotherapy provides an appealing alternative to other therapies, including conventional therapies with lower response rates. However, there are significant regulatory and logistical concerns associated with the ex vivo activation and large-scale expansion of these cells. The best current practice uses a rapid expansion protocol (REP) consisting of an ex vivo process that occurs in tissue culture flasks (T-flasks) and gas-permeable bags, utilizes OKT3 (anti-CD3 monoclonal antibody), recombinant human interleukin-2, and irradiated peripheral blood mononuclear cells to initiate rapid lymphocyte growth. A major limitation to the widespread delivery of therapy to large numbers of melanoma patients is the open system in which a REP is initiated. To address this problem, we have investigated the initiation, expansion and harvest at clinical scale of TIL in a closed-system continuous perfusion bioreactor. Each cell product met all safety criteria for patient treatment and by head-to-head comparison had a similar potency and phenotype as cells grown in control T-flasks and gas-permeable bags. However, the currently available bioreactor cassettes were limited in the total cell numbers that could be generated. This bioreactor may simplify the process of the rapid expansion of TIL under stringent regulatory conditions thereby enabling other institutions to pursue this form of ACT.
Figures


References
-
- Brand KA, Hermfisse U. Aerobic glycolysis by proliferating cells: a protective strategy against reactive oxygen species. FASEB J. 1997;11:388. - PubMed
-
- Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, Wunderlich J, Restifo NP, Thomasian A, Downey SG, Smith FO, Klapper J, Morton K, Laurencot C, White DE, Rosenberg SA. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233. - PMC - PubMed
-
- Gattinoni L, Klebanoff CA, Palmer DC, Wrzesinski C, Kerstann K, Yu Z, Finkelstein SE, Theoret MR, Rosenberg SA, Restifo NP. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616. - PMC - PubMed
-
- Guardino AE, Rajapaksa R, Ong KH, Sheehan K, Levy R. Production of myeloid dendritic cells (DC) pulsed with tumor-specific idiotype protein for vaccination of patients with multiple myeloma. Cytotherapy. 2006;8:277. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

