Thin-walled microvessels in human coronary atherosclerotic plaques show incomplete endothelial junctions relevance of compromised structural integrity for intraplaque microvascular leakage
- PMID: 19389562
- PMCID: PMC2756458
- DOI: 10.1016/j.jacc.2008.12.056
Thin-walled microvessels in human coronary atherosclerotic plaques show incomplete endothelial junctions relevance of compromised structural integrity for intraplaque microvascular leakage
Abstract
Objectives: This study sought to examine the ultrastructure of microvessels in normal and atherosclerotic coronary arteries and its association with plaque phenotype.
Background: Microvessels in atherosclerotic plaques are an entry point for inflammatory and red blood cells; yet, there are limited data on the ultrastructural integrity of microvessels in human atherosclerosis.
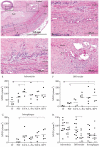
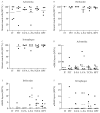
Methods: Microvessel density (MVD) and ultrastructural morphology were determined in the adventitia, intima-media border, and atherosclerotic plaque of 28 coronary arteries using immunohistochemistry for endothelial cells (Ulex europeaus, CD31/CD34), basement membrane (laminin, collagen IV), and mural cells (desmin, alpha-smooth muscle [SM] actin, smoothelin, SM1, SM2, SMemb). Ultrastructural characterization of microvessel morphology was performed by electron microscopy.
Results: The MVD was increased in advanced plaques compared with early plaques, which correlated with lesion morphology. Adventitial MVD was higher than intraplaque MVD in normal arteries and early plaques, but adventitial and intraplaque MVD were similar in advanced plaques. Although microvessel basement membranes were intact, the percentage of thin-walled microvessels was similarly low in normal and atherosclerotic adventitia, in the adventitia and the plaque, and in all plaque types. Intraplaque microvascular endothelial cells (ECs) were abnormal, with membrane blebs, intracytoplasmic vacuoles, open EC-EC junctions, and basement membrane detachment. Leukocyte infiltration was frequently observed by electron microscopy, and confirmed by CD45RO and CD68 immunohistochemistry.
Conclusions: The MVD was associated with coronary plaque progression and morphology. Microvessels were thin-walled in normal and atherosclerotic arteries, and the compromised structural integrity of microvascular endothelium may explain the microvascular leakage responsible for intraplaque hemorrhage in advanced human coronary atherosclerosis.
Figures



Comment in
-
Intrusion through the fragile back door: immature plaque microvessels as entry portals for leukocytes and erythrocytes in atherosclerosis.J Am Coll Cardiol. 2009 Apr 28;53(17):1528-31. doi: 10.1016/j.jacc.2009.01.047. J Am Coll Cardiol. 2009. PMID: 19389563 No abstract available.
References
-
- Moreno PR, Purushothaman KR, Fuster V, et al. Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta: implications for plaque vulnerability. Circulation. 2004;110:2032–8. - PubMed
-
- Virmani R, Kolodgie FD, Burke AP, et al. Atherosclerotic Plaque Progression and Vulnerability to Rupture Angiogenesis as a Source of Intraplaque Hemorrhage. Arterioscler Thromb Vasc Biol. 2005;25:2054–61. - PubMed
-
- Bot I, de Jager SC, Zernecke A, et al. Perivascular mast cells promote atherogenesis and induce plaque destabilization in apolipoprotein E-deficient mice. Circulation. 2007;115:2516–25. - PubMed
-
- Kockx MM, Cromheeke KM, Knaapen MW, et al. Phagocytosis and macrophage activation associated with hemorrhagic microvessels in human atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;23:440–6. - PubMed
-
- Kolodgie FD, Gold HK, Burke AP, et al. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med. 2003;349:2316–25. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

