Identification and functional characterization of protein kinase A-catalyzed phosphorylation of potassium channel Kv1.2 at serine 449
- PMID: 19389710
- PMCID: PMC2713528
- DOI: 10.1074/jbc.M109.010918
Identification and functional characterization of protein kinase A-catalyzed phosphorylation of potassium channel Kv1.2 at serine 449
Abstract
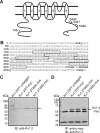
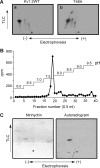
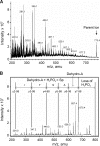
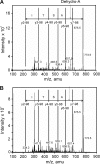
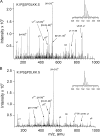
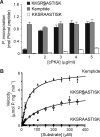
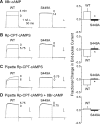
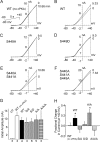
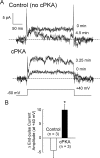
Vascular smooth muscle Kv1 delayed rectifier K+ channels (KDR) containing Kv1.2 control membrane potential and thereby regulate contractility. Vasodilatory agonists acting via protein kinase A (PKA) enhance vascule smooth muscle Kv1 activity, but the molecular basis of this regulation is uncertain. We characterized the role of a C-terminal phosphorylation site, Ser-449, in Kv1.2 expressed in HEK 293 cells by biochemical and electrophysiological methods. We found that 1) in vitro phosphorylation of Kv1.2 occurred exclusively at serine residues, 2) one major phosphopeptide that co-migrated with 449pSASTISK was generated by proteolysis of in vitro phosphorylated Kv1.2, 3) the peptide 445KKSRSASTISK exhibited stoichiometric phosphorylation by PKA in vitro, 4) matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectroscopy (MS) and MS/MS confirmed in vitro Ser-449 phosphorylation by PKA, 5) in situ phosphorylation at Ser-449 was detected in HEK 293 cells by MALDI-TOF MS followed by MS/MS. MIDAS (multiple reaction monitoring-initiated detection and sequencing) analysis revealed additional phosphorylated residues, Ser-440 and Ser-441, 6) in vitro 32P incorporation was significantly reduced in Kv1.2-S449A, Kv1.2-S449D, and Kv1.2-S440A/S441A/S449A mutant channels, but Kv1.2-S440A/S441A was identical to wild-type Kv1.2 (Kv1.2-WT), and 7) bath applied 8-Br-cAMP or dialysis with PKA catalytic subunit (cPKA) increased Kv1.2-WT but not Kv1.2-S449A current amplitude. cPKA increased Kv1.2-WT current in inside-out patches. Rp-CPT-cAMPS reduced Kv1.2-WT current, blocked the increase due to 8-Br-cAMP, but had no effect on Kv1.2-S449A. cPKA increased current due to double mutant Kv1.2-S440A/S441A but had no effect on Kv1.2-S449D or Kv1.2-S440A/S441A/S449A. We conclude that Ser-449 in Kv1.2 is a site of PKA phosphorylation and a potential molecular mechanism for Kv1-containing KDR channel modulation by agonists via PKA activation.
Figures



References
-
- Nelson M. T., Quayle J. M. ( 1995) Am. J. Physiol. 268, C799– C822 - PubMed
-
- Knot H. J., Nelson M. T. ( 1995) Am. J. Physiol. 269, H348– H355 - PubMed
-
- Davis M. J., Hill M. A. ( 1999) Physiol. Rev. 79, 387– 423 - PubMed
-
- Kerr P. M., Clément-Chomienne O., Thorneloe K. S., Chen T. T., Ishii K., Sontag D. P., Walsh M. P., Cole W. C. ( 2001) Circ. Res. 89, 1038– 1044 - PubMed
-
- Thorneloe K. S., Chen T. T., Kerr P. M., Grier E. F., Horowitz B., Cole W. C., Walsh M. P. ( 2001) Circ. Res. 89, 1030– 1037 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

