Adipose triglyceride lipase is implicated in fuel- and non-fuel-stimulated insulin secretion
- PMID: 19389712
- PMCID: PMC2719321
- DOI: 10.1074/jbc.M109.006650
Adipose triglyceride lipase is implicated in fuel- and non-fuel-stimulated insulin secretion
Abstract
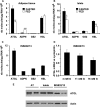
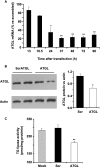
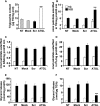
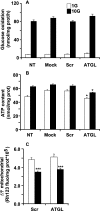
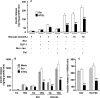
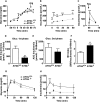
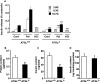
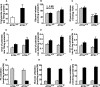
Reduced lipolysis in hormone-sensitive lipase-deficient mice is associated with impaired glucose-stimulated insulin secretion (GSIS), suggesting that endogenous beta-cell lipid stores provide signaling molecules for insulin release. Measurements of lipolysis and triglyceride (TG) lipase activity in islets from HSL(-/-) mice indicated the presence of other TG lipase(s) in the beta-cell. Using real time-quantitative PCR, adipose triglyceride lipase (ATGL) was found to be the most abundant TG lipase in rat islets and INS832/13 cells. To assess its role in insulin secretion, ATGL expression was decreased in INS832/13 cells (ATGL-knockdown (KD)) by small hairpin RNA. ATGL-KD increased the esterification of free fatty acid (FFA) into TG. ATGL-KD cells showed decreased glucose- or Gln + Leu-induced insulin release, as well as reduced response to KCl or palmitate at high, but not low, glucose. The K(ATP)-independent/amplification pathway of GSIS was considerably reduced in ATGL-KD cells. ATGL(-/-) mice were hypoinsulinemic and hypoglycemic and showed decreased plasma TG and FFAs. A hyperglycemic clamp revealed increased insulin sensitivity and decreased GSIS and arginine-induced insulin secretion in ATGL(-/-) mice. Accordingly, isolated islets from ATGL(-/-) mice showed reduced insulin secretion in response to glucose, glucose + palmitate, and KCl. Islet TG content and FFA esterification into TG were increased by 2-fold in ATGL(-/-) islets, but glucose usage and oxidation were unaltered. The results demonstrate the importance of ATGL and intracellular lipid signaling for fuel- and non-fuel-induced insulin secretion.
Figures
Similar articles
-
Hormone-sensitive lipase has a role in lipid signaling for insulin secretion but is nonessential for the incretin action of glucagon-like peptide 1.Diabetes. 2004 Jul;53(7):1733-42. doi: 10.2337/diabetes.53.7.1733. Diabetes. 2004. PMID: 15220197
-
Hepatic overexpression of hormone-sensitive lipase and adipose triglyceride lipase promotes fatty acid oxidation, stimulates direct release of free fatty acids, and ameliorates steatosis.J Biol Chem. 2008 May 9;283(19):13087-99. doi: 10.1074/jbc.M800533200. Epub 2008 Mar 12. J Biol Chem. 2008. PMID: 18337240 Free PMC article.
-
Beta cell compensation for insulin resistance in Zucker fatty rats: increased lipolysis and fatty acid signalling.Diabetologia. 2006 Sep;49(9):2120-30. doi: 10.1007/s00125-006-0305-5. Epub 2006 Jul 26. Diabetologia. 2006. PMID: 16868750
-
Fate of fat: the role of adipose triglyceride lipase in lipolysis.Biochim Biophys Acta. 2009 Jun;1791(6):494-500. doi: 10.1016/j.bbalip.2008.10.005. Epub 2008 Oct 29. Biochim Biophys Acta. 2009. PMID: 19010445 Review.
-
The role of adipose triglyceride lipase in lipid and glucose homeostasis: lessons from transgenic mice.Lipids Health Dis. 2019 Nov 22;18(1):204. doi: 10.1186/s12944-019-1151-z. Lipids Health Dis. 2019. PMID: 31757217 Free PMC article. Review.
Cited by
-
Urea impairs β cell glycolysis and insulin secretion in chronic kidney disease.J Clin Invest. 2016 Sep 1;126(9):3598-612. doi: 10.1172/JCI86181. Epub 2016 Aug 15. J Clin Invest. 2016. PMID: 27525435 Free PMC article.
-
Desnutrin/ATGL activates PPARδ to promote mitochondrial function for insulin secretion in islet β cells.Cell Metab. 2013 Dec 3;18(6):883-95. doi: 10.1016/j.cmet.2013.10.012. Epub 2013 Nov 21. Cell Metab. 2013. PMID: 24268737 Free PMC article.
-
Contribution of Mitochondria to Insulin Secretion by Various Secretagogues.Antioxid Redox Signal. 2022 May;36(13-15):920-952. doi: 10.1089/ars.2021.0113. Epub 2021 Aug 24. Antioxid Redox Signal. 2022. PMID: 34180254 Free PMC article. Review.
-
Lysophosphatidic acid impairs glucose homeostasis and inhibits insulin secretion in high-fat diet obese mice.Diabetologia. 2013 Jun;56(6):1394-402. doi: 10.1007/s00125-013-2891-3. Epub 2013 Mar 19. Diabetologia. 2013. PMID: 23508306
-
Differential Insulin Secretion of High-Fat Diet-Fed C57BL/6NN and C57BL/6NJ Mice: Implications of Mixed Genetic Background in Metabolic Studies.PLoS One. 2016 Jul 12;11(7):e0159165. doi: 10.1371/journal.pone.0159165. eCollection 2016. PLoS One. 2016. PMID: 27403868 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

