Immunoregulatory functions of mTOR inhibition
- PMID: 19390566
- PMCID: PMC2847476
- DOI: 10.1038/nri2546
Immunoregulatory functions of mTOR inhibition
Abstract
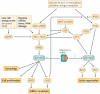
The potent immunosuppressive action of rapamycin is commonly ascribed to inhibition of growth factor-induced T cell proliferation. However, it is now evident that the serine/threonine protein kinase mammalian target of rapamycin (mTOR) has an important role in the modulation of both innate and adaptive immune responses. mTOR regulates diverse functions of professional antigen-presenting cells, such as dendritic cells (DCs), and has important roles in the activation of effector T cells and the function and proliferation of regulatory T cells. In this Review, we discuss our current understanding of the mTOR pathway and the consequences of mTOR inhibition, both in DCs and T cells, including new data on the regulation of forkhead box P3 expression.
Figures



References
-
- Vezina C, Kudelski A, Sehgal SN. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J. Antibiot. (Tokyo) 1975;28:721–726. - PubMed
-
- Saunders RN, Metcalfe MS, Nicholson ML. Rapamycin in transplantation: a review of the evidence. Kidney Int. 2001;59:3–16. - PubMed
-
- Eisen HJ, et al. Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant recipients. N. Engl. .J Med. 2003;349:847–858. - PubMed
-
- Sabatini DM. mTOR and cancer: insights into a complex relationship. Nature Rev. Cancer. 2006;6:729–734. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

