Control of metabolic homeostasis by stress signaling is mediated by the lipocalin NLaz
- PMID: 19390610
- PMCID: PMC2667264
- DOI: 10.1371/journal.pgen.1000460
Control of metabolic homeostasis by stress signaling is mediated by the lipocalin NLaz
Abstract
Metabolic homeostasis in metazoans is regulated by endocrine control of insulin/IGF signaling (IIS) activity. Stress and inflammatory signaling pathways--such as Jun-N-terminal Kinase (JNK) signaling--repress IIS, curtailing anabolic processes to promote stress tolerance and extend lifespan. While this interaction constitutes an adaptive response that allows managing energy resources under stress conditions, excessive JNK activity in adipose tissue of vertebrates has been found to cause insulin resistance, promoting type II diabetes. Thus, the interaction between JNK and IIS has to be tightly regulated to ensure proper metabolic adaptation to environmental challenges. Here, we identify a new regulatory mechanism by which JNK influences metabolism systemically. We show that JNK signaling is required for metabolic homeostasis in flies and that this function is mediated by the Drosophila Lipocalin family member Neural Lazarillo (NLaz), a homologue of vertebrate Apolipoprotein D (ApoD) and Retinol Binding Protein 4 (RBP4). Lipocalins are emerging as central regulators of peripheral insulin sensitivity and have been implicated in metabolic diseases. NLaz is transcriptionally regulated by JNK signaling and is required for JNK-mediated stress and starvation tolerance. Loss of NLaz function reduces stress resistance and lifespan, while its over-expression represses growth, promotes stress tolerance and extends lifespan--phenotypes that are consistent with reduced IIS activity. Accordingly, we find that NLaz represses IIS activity in larvae and adult flies. Our results show that JNK-NLaz signaling antagonizes IIS and is critical for metabolic adaptation of the organism to environmental challenges. The JNK pathway and Lipocalins are structurally and functionally conserved, suggesting that similar interactions represent an evolutionarily conserved system for the control of metabolic homeostasis.
Conflict of interest statement
The authors have declared that no competing interests exist.
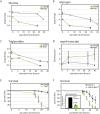
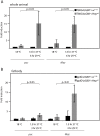
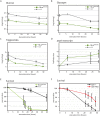
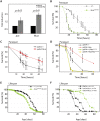
Figures


References
-
- Kloting N, Bluher M. Extended longevity and insulin signaling in adipose tissue. Exp Gerontol. 2005;40:126–133. - PubMed
-
- Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. - PubMed
-
- Russell SJ, Kahn CR. Endocrine regulation of ageing. Nat Rev Mol Cell Biol. 2007;8:681–691. - PubMed
-
- Wang MC, Bohmann D, Jasper H. JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell. 2005;121:115–125. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

